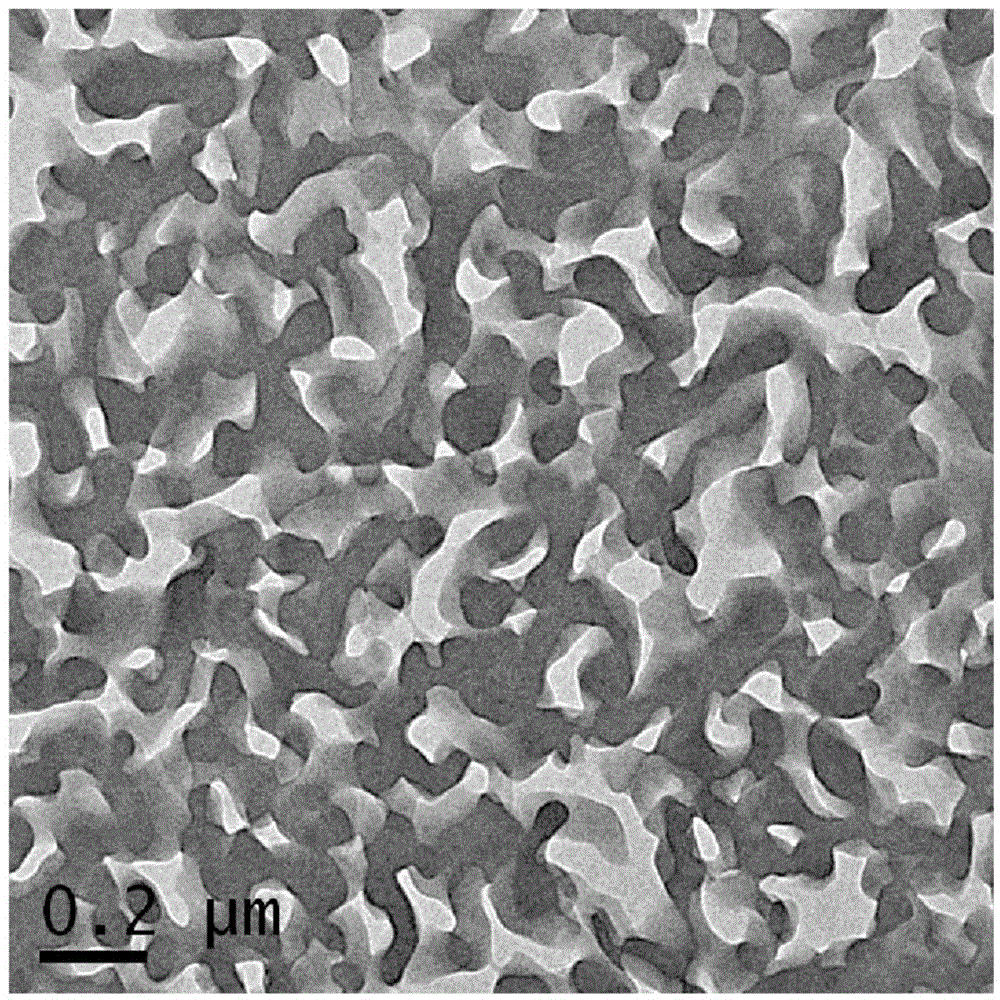
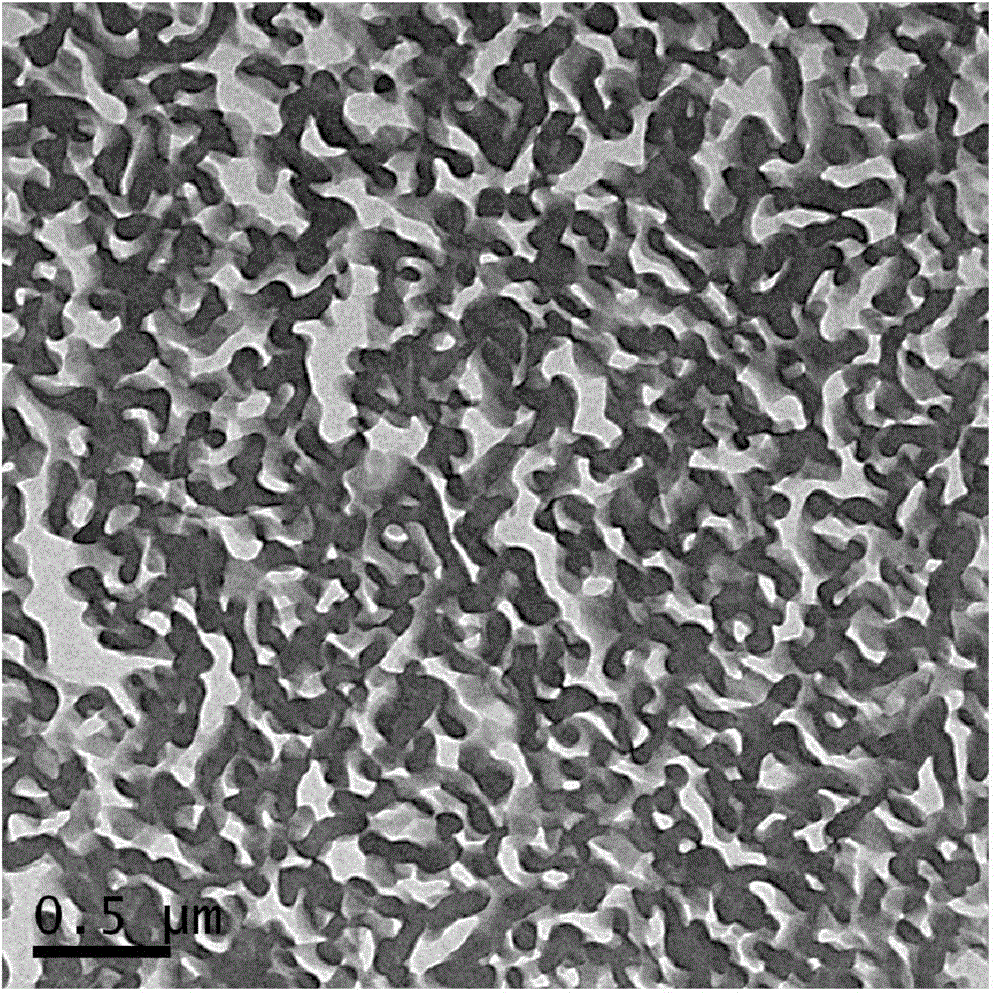
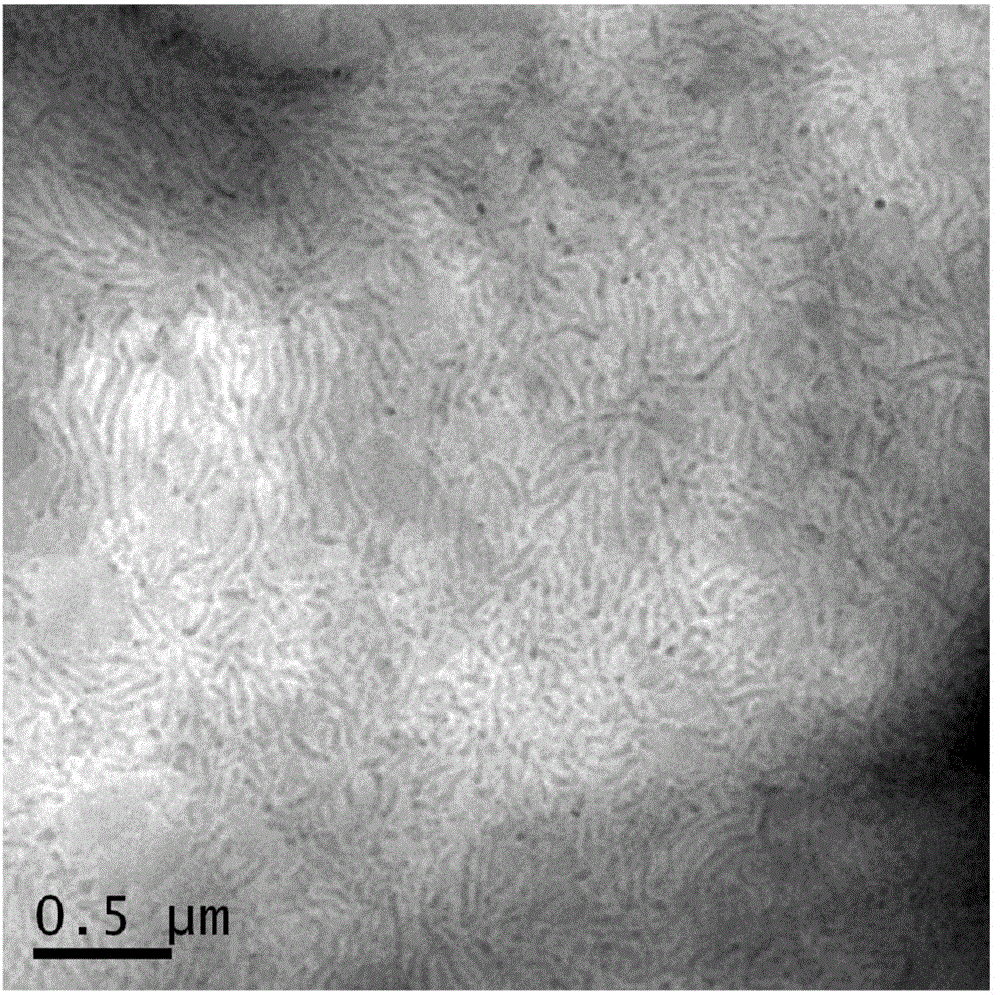
Multi-block copolymer and polymer electrolyte
A multi-block copolymer and polymer technology, which is applied in the field of polymer electrolyte materials, can solve the long-term durability requirements and industrial applications of lithium-ion batteries without materials, which cannot meet the requirements of electrolyte thin films, poor mechanical properties of polymer electrolyte membranes, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 (Preparation of A-B-A-B type tetrablock copolymer and corresponding polymer electrolyte, wherein poly(poly(ethylene glycol) methyl ether methacrylate) (expressed as P(PEGMA)) is used as segment A and the use of polystyrene (denoted PS) as segment B)
[0089] (1) Synthesis of Segment A
[0090] Add 15.5 g poly(ethylene glycol) methyl ether methacrylate (PEGMA-1, M n =980, m=20, 15.8mmol) and in N 2 Dissolve under atmosphere. Then 45.3 mg (0.316 mmol) CuBr and 98.65 mg (0.732 mmol) bpy were added to the solution. After stirring well for 10 minutes, 57.2 mg (0.316 mmol) of ethyl 2-bromopropionate was added, and the temperature was raised to 90°C. After 30 hours of polymerization, the reaction system was immediately cooled in an ice / water bath to terminate the reaction. The solution was then diluted with THF and passed through Al 2 o 3 The column was purified to remove the catalyst / ligand complex. After removing the solvent, the reaction product was washed...
Embodiment 2
[0103] Example 2 (Preparation of A-B-A-B-A type pentablock copolymer and corresponding polymer electrolyte, wherein P (PEGMA) is used as segment A and PS is used as segment B)
[0104] The P(PEGMA)-b-PS-b-P(PEGMA)-b-PS tetrablock copolymer (A-B-A-B type) prepared in Example 1 was used as a macroinitiator to prepare a pentablock copolymer.
[0105] (1) Synthesis of A-B-A-B-A type pentablock copolymer
[0106] Under an argon atmosphere, 10.92 mg (0.075 mmol) of CuBr and 23.77 mg (0.15 mmol) of bpy were added to 5.2 ml of toluene to form a reaction system. After stirring for 10 minutes, a catalyst / ligand complex was formed. Add 3.13g (3.0mmol) PEGMA-1 (M n =980, m=20), stirring and dissolving was continued for 15 minutes under an argon atmosphere. Next, the reaction system was sealed, and after the macromolecular initiator was completely dissolved, the reaction temperature was raised to 90°C. Polymerization was carried out for 100 hours. Immediately thereafter, the reaction ...
Embodiment 3
[0114] Example 3 (Preparation of A-B-A-B-A type pentablock copolymer and corresponding polymer electrolyte, wherein P (PEGMA) is used as segment A and PS is used as segment B)
[0115] (1) Synthesis of Segment A
[0116] In 25ml toluene, add 18.0g PEGMA-2 (M n =450, m=8, 40.0mmol) and in N 2 Dissolve under atmosphere. Then 57.34 mg (0.40 mmol) CuBr and 107.82 mg (0.80 mmol) bpy were added to the solution. After stirring well for 10 minutes, 75.57 mg (0.40 mmol) of 2,2-dichloroacetophenone was added, and the temperature was raised to 90°C. After 25 hours of polymerization, the reaction system was immediately cooled in an ice / water bath to terminate the reaction. The solution was then diluted with THF and passed through Al 2 o 3The column was purified to remove the catalyst / ligand complex. After removing the solvent, the reaction product was washed with anhydrous diethyl ether to remove unreacted monomers to obtain refined P(PEGMA)-2. M of P(PEGMA)-2 n 6000 and molecula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com