Tenofovir disoproxil fumarate tablet that is easy to dissolve and preparation method thereof
A technology of tenofovir fumarate and disoproxil, which is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc., can solve the problem of reducing the biological activity of new drug compounds. Affecting drug absorption and metabolism process, unfavorable to bioavailability and other problems, to achieve the effect of improving bioavailability, efficient and fast process, and uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
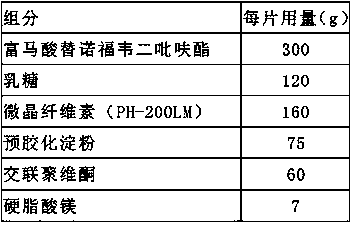
Embodiment 1
[0034] Using fluidized bed granulation and tableting process:
[0035]Preparation method: weigh tenofovir disoproxil fumarate, lactose, pregelatinized starch, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, Cellactose80, tenofovir fumarate Fovir dipivoxil, lactose, pregelatinized starch, and microcrystalline cellulose are passed through a 100-mesh sieve, croscarmellose sodium is passed through a 80-mesh sieve, and magnesium stearate is passed through a 60-mesh sieve. Mix tenofovir disoproxil fumarate, lactose, pregelatinized starch, microcrystalline cellulose and croscarmellose sodium added inside, and prepare in a fluidized bed with water as a wetting agent Granules, the air inlet temperature is controlled at 45°C to 55°C. After granulation, add additional croscarmellose sodium Cellactose80 and magnesium stearate to mix evenly, detect the content of the intermediate, and determine the tablet weight according to the content, and adjust the tableting pre...
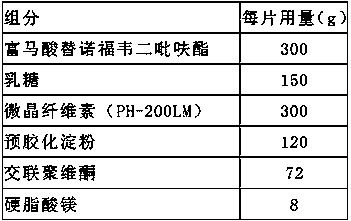
Embodiment 2
[0037] Using fluidized bed granulation and tableting process:
[0038] Preparation method: weigh tenofovir disoproxil fumarate, lactose, pregelatinized starch, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, Cellactose80, tenofovir fumarate Fovir dipivoxil, lactose, pregelatinized starch, and microcrystalline cellulose are passed through a 100-mesh sieve, croscarmellose sodium is passed through a 80-mesh sieve, and magnesium stearate is passed through a 60-mesh sieve. Mix tenofovir disoproxil fumarate, lactose, pregelatinized starch, microcrystalline cellulose and croscarmellose sodium added inside, and prepare in a fluidized bed with water as a wetting agent Granules, the air inlet temperature is controlled at 45°C to 55°C. After granulation, add additional croscarmellose sodium Cellactose80 and magnesium stearate to mix evenly, detect the content of the intermediate, and determine the tablet weight according to the content, and adjust the tableting pr...
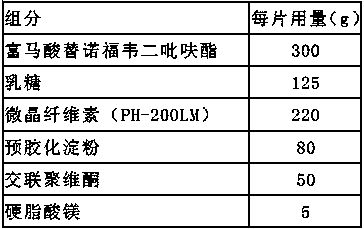
Embodiment 3
[0040] Using fluidized bed granulation and tableting process:
[0041] Preparation method: weigh tenofovir disoproxil fumarate, lactose, pregelatinized starch, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, Cellactose80, tenofovir fumarate Fovir dipivoxil, lactose, pregelatinized starch, and microcrystalline cellulose are passed through a 100-mesh sieve, croscarmellose sodium is passed through a 80-mesh sieve, and magnesium stearate is passed through a 60-mesh sieve. Mix tenofovir disoproxil fumarate, lactose, pregelatinized starch, microcrystalline cellulose and croscarmellose sodium added inside, and prepare in a fluidized bed with water as a wetting agent Granules, the air inlet temperature is controlled at 45°C to 55°C. After granulation, add additional croscarmellose sodium Cellactose80 and magnesium stearate to mix evenly, detect the content of the intermediate, and determine the tablet weight according to the content, and adjust the tableting pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com