Method for preparing newcastle disease inactivated vaccine, and product and application thereof
An inactivated vaccine and Newcastle disease technology, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, virus antigen components, etc., can solve the problems of producing Newcastle disease inactivated vaccines in cell factories, and meet the requirements of immune production and reduce the size of the vaccine. Batch-to-batch differences and the effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of Newcastle Disease Inactivated Vaccine by Propagating Newcastle Disease Virus in Cell Factory
[0034] 1. Experimental method
[0035] 1.1 Cell culture
[0036] First, resuscitate the seeded cells DF1 into T75 cell flasks, grow for about 48 hours, and pass passage according to the ratio of 1:4; then transfer the cells in 6 T75 cell flasks full of cells to a 10L spinner bottle for culture; about 48 hours Subculture the cells at a ratio of 1:3; the medium used in this process is DMEM, the serum is fetal bovine serum, and the usage amount is 10%.
[0037] The cells obtained in the spinner bottle were washed twice with PBS, and then 125 ml of 0.25% trypsin-EDTA solution was added for digestion, and the cells in the spinner bottle were harvested.
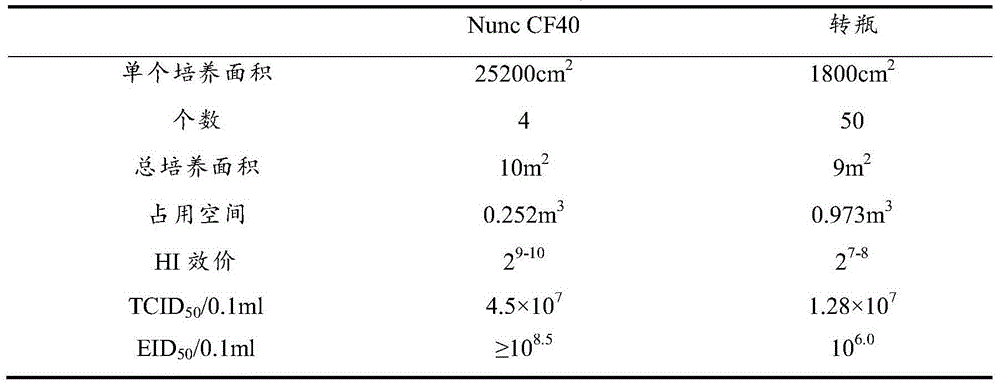
[0038] After the harvested cells were counted, the DF1 cells were cultured in the Thermo Scientific Nunc cell factory, and the medium used was DMEM medium containing 10% fetal bovine serum. Samples were ...
experiment example 1
[0081] Experimental Example 1 Optimization of Newcastle Disease Virus Proliferation Conditions in Cell Factory
[0082] 1. Experimental method
[0083] 1.1 The effect of cell density on the proliferation of Newcastle disease virus
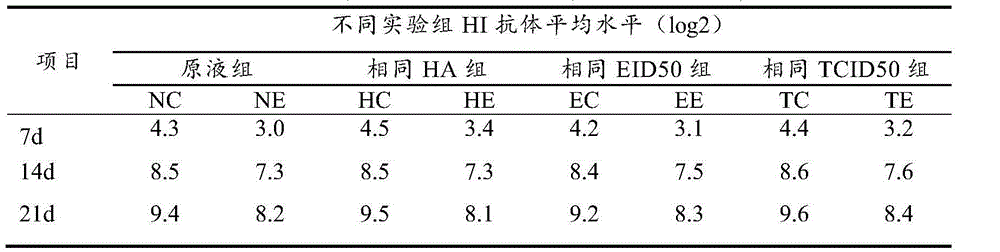
[0084] At different cell densities (Table 4), the virus was inoculated at MOI=0.2, cultured at 37°C, and the virus was harvested at different time points, and the difference in NDV virus hemagglutination (HA) under different proliferation conditions was compared. The results are shown in Table 4.
[0085] Table 4 Hemagglutination value of virus at different times after inoculation with different cell densities (log2)
[0086]
[0087] As can be seen from Table 4, the cell density was 0.9×10 5 When cells / ml, cultured at 37°C for 48-72h, NDV virus HA reached 8.2log2, better than other treatments. Therefore, the present invention utilizes the cell density of cell factory propagation NDV virus to be determined as 0.9 * 10 5 cells / ml.
[0088] 1...
experiment example 2
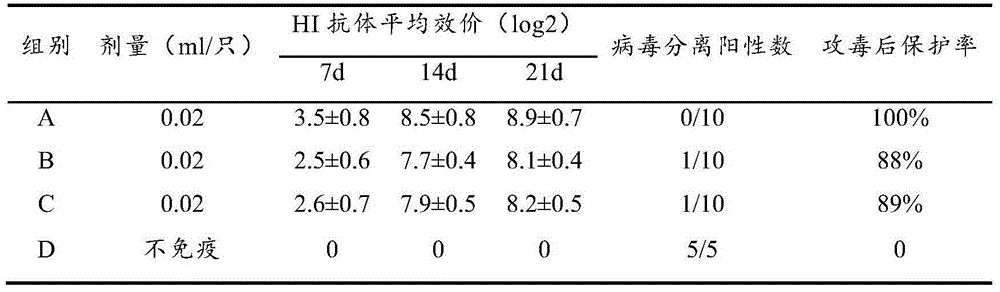
[0099] Experimental Example 2 Preparation of Newcastle Disease Inactivated Vaccine with Different Adjuvants and Experiment of Immunity Effect
[0100] 1. Experimental methods and results of adjuvant component screening
[0101] The improved adjuvant A is composed of levamisole, chitosan and complete Freund's adjuvant, 5 mg of levamisole and 8 mg of chitosan in each milliliter of adjuvant, and the balance is complete Freund's adjuvant;
[0102] The improved adjuvant B is composed of levamisole, white oil and saponin, 5 mg of levamisole and 8 mg of saponin in each milliliter of adjuvant, and the balance is ordinary white oil;
[0103] The improved adjuvant C is composed of white oil, chitosan and carbomer (resin), 5 mg of chitosan and 8 mg of carbomer per milliliter of adjuvant, and the balance is ordinary white oil;
[0104] The improved adjuvant D is composed of levamisole, chitosan and white oil. Each milliliter of adjuvant contains 5 mg of levamisole and 8 mg of chitosan, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com