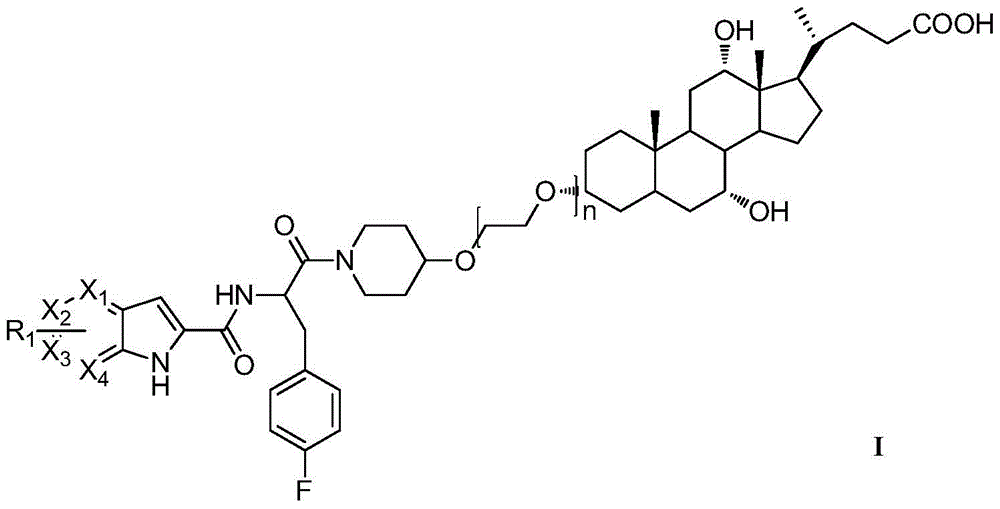
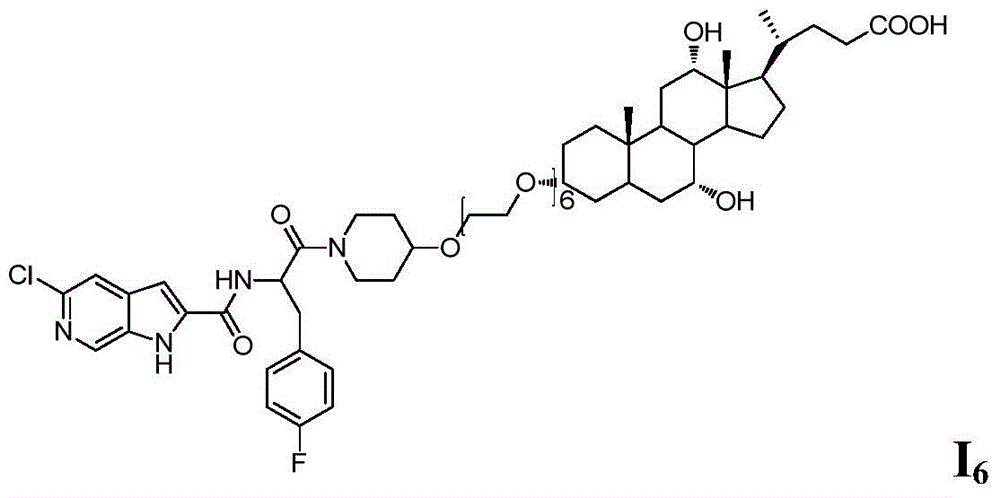
A kind of compound and its preparation method and application
A compound and catalyst technology, which is applied in the field of bile acid-conjugated pyrroleamide glycogen phosphorylase inhibitors, can solve the problems of direct connection and difficult dissociation of chemical bonds, and achieve the effect of good GP inhibitory activity and favorable activity maintenance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
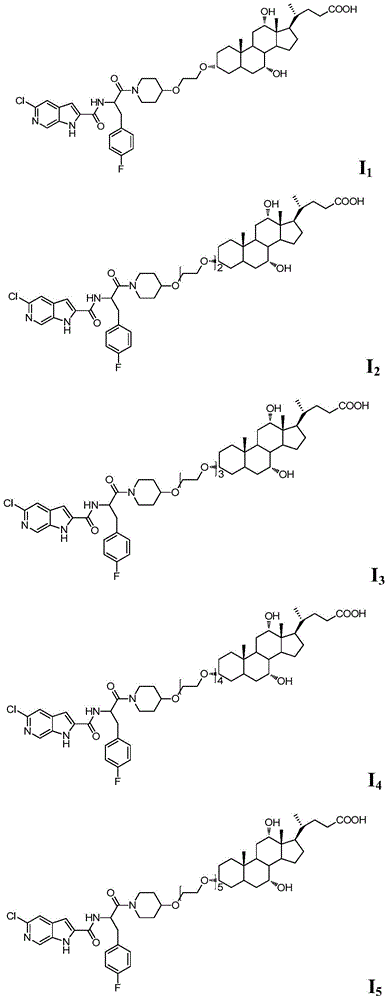
Embodiment 1
[0040] Compound I 1 Preparation
[0041]
[0042] 3α-Methanesulfonyloxy-7α,12α-Dihydroxy-5β-cholestane-24-carboxylic acid methyl ester(III)
[0043] Under ice bath, dissolve methyl cholate (0.19g, 0.66mol) in pyridine (10mL), add p-toluenesulfonyl chloride (97mg, 0.79mmol) and DMAP (97mg, 0.79mmol) with stirring, and stir overnight at room temperature. The next day, it was concentrated under reduced pressure, the residue was diluted with ethyl acetate, washed with saturated brine, and anhydrous Na 2 SO 4 Dry, evaporate the solvent under reduced pressure, flash column chromatography (petroleum ether / ethyl acetate 1 / 1, V / V) to obtain a white solid (1.09 g, 95%). ESI-MS:500.5[M+H] + ; 1 H NMR(400MHz, CDCl 3 )δ: 0.66 (s, 3H), 0.87 (s, 3H), 0.94 (d, J = 6.3 Hz, 3H), 2.38-1.25 (m, 25H), 2.56-2.52 (m, 1H), 2.48 (s ,3H), 3.68(s,3H), 3.84(s,1H), 7.32(d,J=8.0Hz,2H), 7.79(d,J=8.4Hz,2H).
[0044] 3α-hydroxyethoxy-7α,12α-dihydroxy-5β-cholestane-24-carboxylic acid methyl ester (V 1 )
[0045] 3α-...
Embodiment 2
[0051] Compound I 2 Preparation
[0052]
[0053] 3α-(Hydroxyethoxy)ethoxy-7α,12α-Dihydroxy-5β-cholestane-24-carboxylic acid methyl ester (V 2 )
[0054] Refer to the preparation method of 3α-hydroxyethoxy-7α,12α-dihydroxy-5β-cholestane-24-carboxylic acid methyl ester to obtain a white solid (174 mg, 41%). ESI-MS:511.4[M+H] + ; 1 H NMR(400MHz, CDCl 3 )δ:0.62(s,3H),0.85(s,3H),0.91(d,J=6.4Hz,3H),1.97-1.03(m,21H),2.18-2.03(m,5H),3.47-3.45 (m, 2H), 3.65-3.54 (m, 10H).
[0055] 3α-(sulfonyloxyethoxy)ethoxy-7α,12α-dihydroxy-5β-cholestane-24-carboxylic acid methyl ester (VI 2 )
[0056] Refer to the preparation method of 3α-methanesulfonyloxy-7α,12α-dihydroxy-5β-cholestane-24-carboxylate to obtain a white solid (159mg, 51%). ESI-MS: 682.5[M+18] + ; 1 H NMR(400MHz, CDCl 3 )δ:0.62(s,3H),0.84(s,3H),0.91(d,J=6.0Hz,3H),1.96-1.05(m,22H),2.35-2.03(m,4H),2.37(s ,3H),3.65-3.36(m,10H),3.79(s,1H),7.27(d,J=8.0Hz,2H),7.74(d,J=8.0Hz,2H).
[0057] (3α-Oxyethoxyethoxy-cholic acid-yl)-{1-[2-(5-chloro-1H-py...
Embodiment 3
[0060] Compound I 3 Preparation
[0061]
[0062] 3α-[(hydroxyethoxy)ethoxy]ethoxy-7α,12α-dihydroxy-5β-cholestane-24-carboxylic acid methyl ester (V 3 )
[0063] Refer to the preparation method of 3α-hydroxyethoxy-7α,12α-dihydroxy-5β-cholestane-24-carboxylate to obtain a white solid (516 mg, 24%). ESI-MS: 555.5[M+H] + ; 1 H NMR(400MHz, CDCl 3 )δ: 0.62 (s, 3H), 0.84 (s, 3H), 0.91 (d, J = 5.6 Hz, 3H), 1.98-1.01 (m, 21H), 2.30-2.02 (m, 5H), 2.90-2.65 (s,1H),3.66-3.44(m,16H).
[0064] 3α-[(sulfonyloxyethoxy)ethoxy]ethoxy-7α,12α-dihydroxy-5β-cholestane-24-carboxylic acid methyl ester (VI 3 )
[0065] Refer to the preparation method of 3α-methanesulfonyloxy-7α,12α-dihydroxy-5β-cholestane-24-carboxylate to obtain a white solid (870mg, 27%). ESI-MS:726.5[M+18] + ; 1 H NMR(400MHz, CDCl 3 )δ:0.62(s,3H),0.83(s,3H),0.91(d,J=6.0Hz,3H),1.95-1.05(m,22H),2.35-2.03(m,4H),2.38(s ,3H),3.50(s,1H),3.64-3.41(m,14H),3.78(s,1H),7.27(d,J=8.4Hz,2H),7.73(d,J=8.4Hz,2H) .
[0066] (3α-Oxyethoxyethoxyethoxy-cho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com