Mitochondrial DNA copy index variability detecting device
A detection device and mitochondrial technology, applied in the field of medical detection, can solve the problems of uncertain definition of the measurement unit of chrM, inability to compare and analyze, inconsistent results, etc., and achieve the effects of high stability, difficult misdiagnosis, and small random errors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 A detection device for mitochondrial DNA copy number variability
[0028] This embodiment relates to the structural composition of a detection device for mitochondrial DNA copy number variability. The device includes a sample preparation unit, a sequencing unit, a calculation unit, and a comparison and judgment unit, wherein:
[0029] The sample preparation unit includes disposable venous blood collection needles, disposable vacuum blood collection tubes, blood anticoagulant EDTA, blood cell protection agent, and plasma free DNA purification kit. The sample preparation unit is used to draw peripheral blood from the vein of the subject and separate Get the cell-free DNA in its plasma.
[0030] The sequencing unit includes a quality inspection module, a high-throughput sequencing module, and a data optimization module, and the high-throughput sequencing module further includes a library construction kit and a high-throughput sequencing kit. The quality inspecti...
Embodiment 2
[0036] Embodiment 2 Determination method of the diagnostic limit value in the detection device of embodiment 1
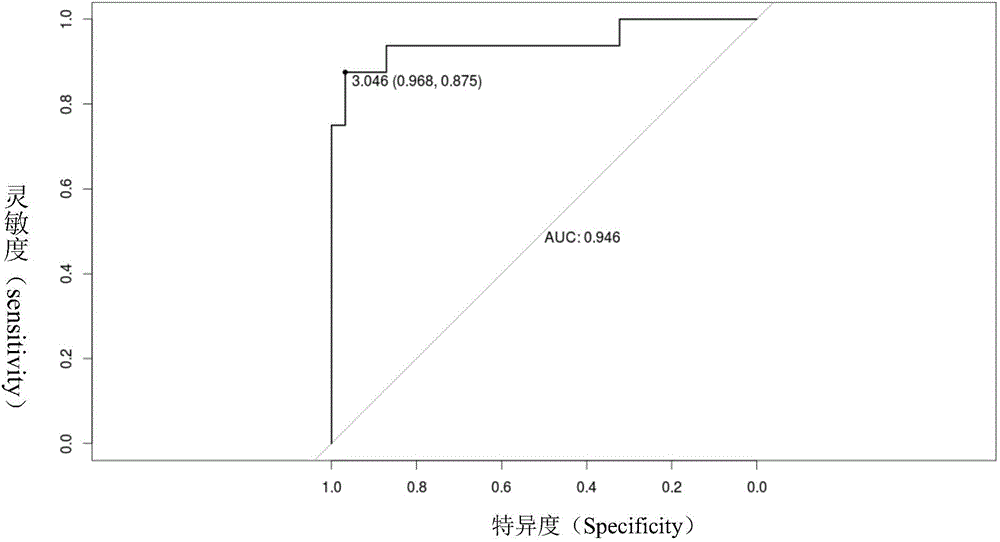
[0037] In the detection device for mitochondrial DNA copy number variability provided in Example 1 of the present invention, the method for obtaining the diagnostic threshold value 3.046 preset by the comparison judgment unit:
[0038] (1) Get a group of (31) free DNA in the plasma of healthy people as a control sample group, carry out whole genome sequencing to these control samples with the same device and method as the sequencing unit in Example 1 of the present invention, each control sample Obtain at least 20M sequencing numbers, compare the sequencing results to the human reference genome, and calculate the relative ratio M of the mitochondrial DNA copy number and the nuclear DNA copy number of each control sample H and M H The natural logarithm of lnM H :
[0039] m H =n H (chrM) / len(chrM) / n H (chrN) / len(chrN),
[0040] where: n H (chrM) is the number...
Embodiment 3 Embodiment 1 test test example
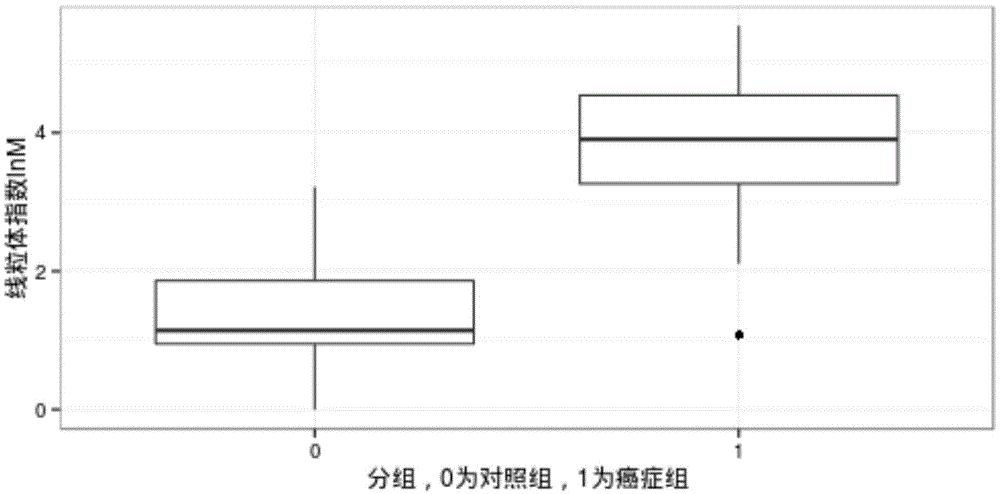
[0059] This embodiment relates to a test example of cancer screening using the mitochondrial DNA copy number variability detection device of embodiment 1.
[0060] 1. Use the sample preparation unit in the detection device to draw 5ml of venous blood from 5 subjects, separate the plasma, and extract free DNA fragments from the plasma as samples to be tested:
[0061] Plasma separation method: Centrifuge 5ml of venous blood at 1000rpm / min for 10 minutes, take out the upper layer of plasma and then centrifuge at 8000rpm / min for 10 minutes, and absorb the upper layer of plasma for DNA extraction;
[0062] The free DNA fragments were extracted using: QIAamp DNA Blood Mini Kit (cat.no.51104), a DNA extraction kit from QIAGEN.
[0063] 2. Use the quality inspection module to perform quality inspection of the concentration and fragment size of the DNA fragments of the sample to be inspected:
[0064] Quality inspection standard: TBS380 is used to quantify the extracted DNA, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com