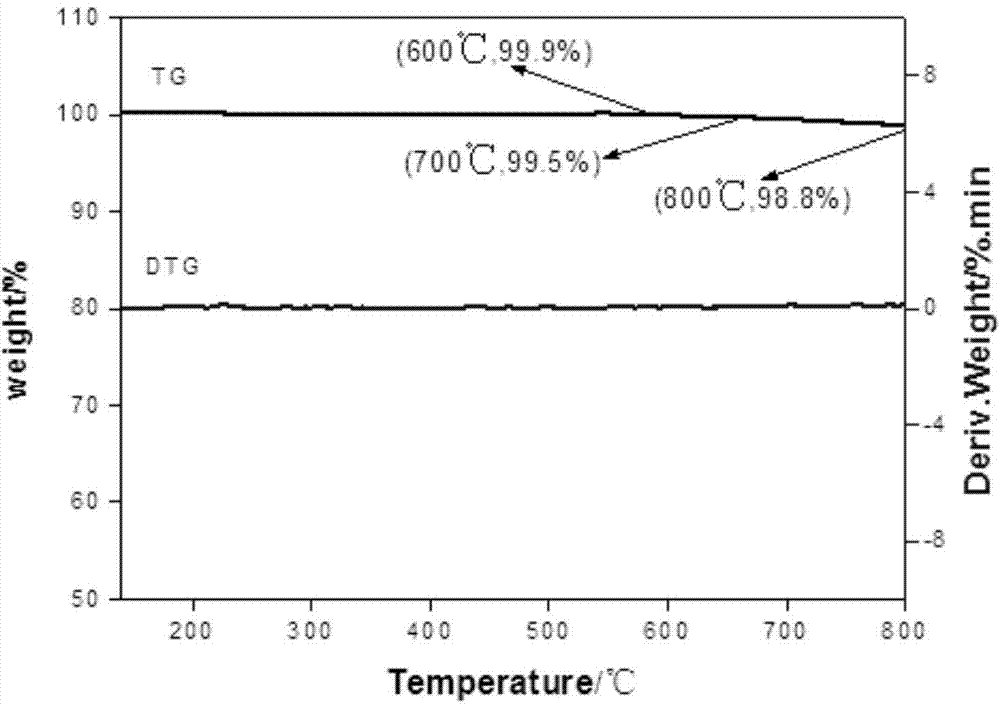
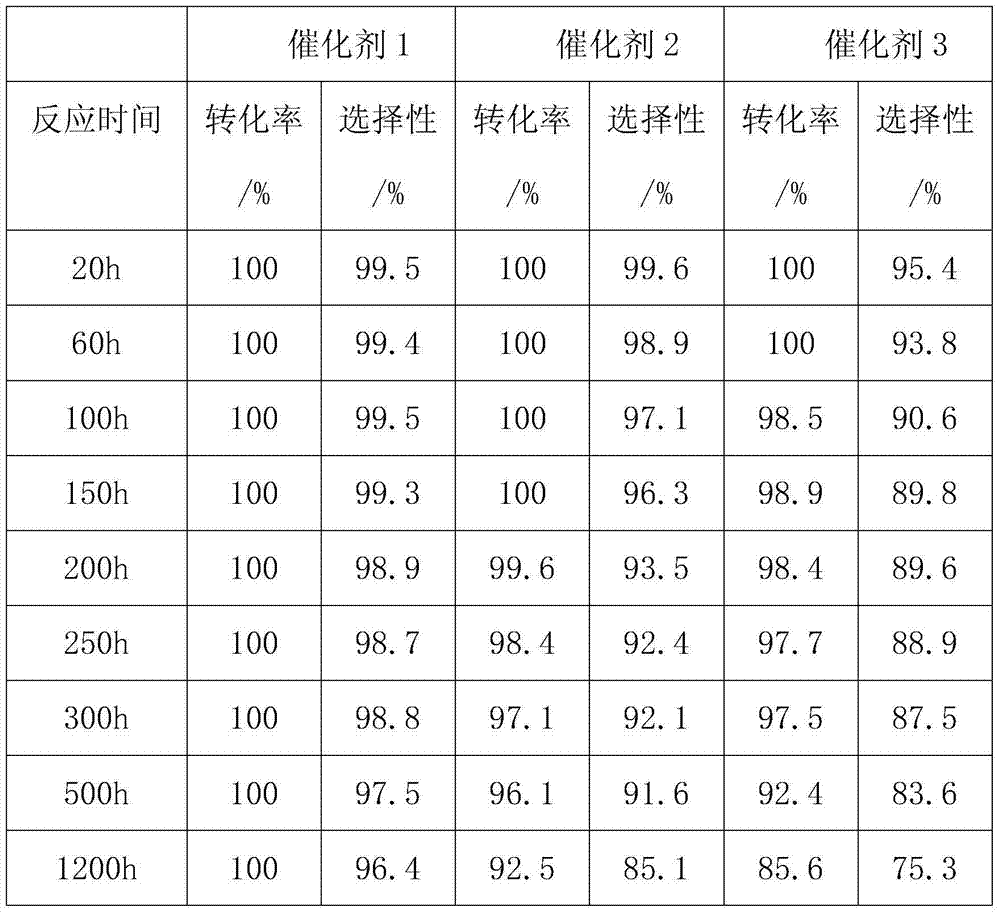
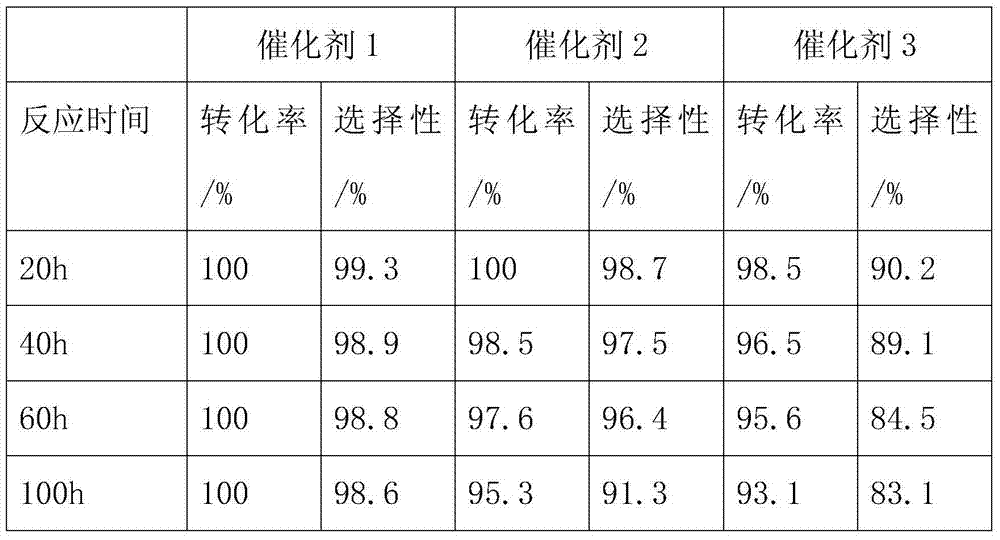
Method for preparing 2-chloro-3,3,3-trifluoropropylene catalyst by gas phase fluorination
A gas-phase fluorination and trifluoropropene technology, applied in the field of chemistry, can solve the problems of low yield, low catalyst service life, complicated reaction process, etc., and achieves simple preparation process, good service life, regeneration performance, repeatability, etc. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Catalyst preparation:
[0027] 83.5g of Fe(NO 3 ) 3 9H 2 O and 317.9g of Mg(NO 3 ) 2 ·6H 2 O is formulated into a mixed solution according to the molar ratio of Fe and Mg elements of 1:6, heated to 40°C, adding 124g of urea and a certain amount of ammonia water to it, adjusting the pH value to 9.5, aging for 1h, and raising the temperature to 90°C. Continue to age for 8 hours, filter, wash the resulting colloidal precipitate to neutrality, and dry at 120°C. The obtained solid is calcined at 500°C for 6 hours to obtain Fe-Mg composite oxide.
[0028] Take 100g of Fe-Mg composite oxide and 25g of magnesium carbonate, mix according to the mass ratio of Fe-Mg composite oxide and magnesium carbonate of 4:1, fully grind with a ball mill, and then make a catalyst precursor by tableting. The catalyst precursor was calcined in a muffle furnace at 400°C for 8 hours, then 100ml of the catalyst precursor was loaded into a tubular reactor, the temperature was lowered to 200°C,...
Embodiment 2
[0032] Catalyst preparation:
[0033] 55.9g of FeCl 3 ·6H 2 O and 252.3 g of MgCl 2 ·6H 2 O is formulated into a mixed solution according to the molar ratio of Fe and Mg elements of 1:6, heated to 40°C, adding 124g of urea and a certain amount of NaOH to it, adjusting the pH value to 8.5, aging for 2h, and raising the temperature to 90°C. Continue to age for 12 hours, filter, wash the obtained colloidal precipitated product to neutrality, dry at 120°C, and roast the obtained solid at 450°C for 6 hours to obtain Fe-Mg composite oxide.
[0034] Evaluation of the catalyst was carried out in the same manner as in Example 1. After the reaction product was washed with water and alkali to remove HCl and HF, the conversion rate of 1,1,2,3-tetrachloropropene was 100% and the selectivity of HCFC-1233xf was 98.3% according to gas chromatography.
Embodiment 3
[0036] The preparation and evaluation of the catalyst of this embodiment are carried out according to the same method as in Example 1, except that 83.5g of Fe(NO 3 ) 3 9H 2 O and 317.9g of Mg(NO 3 ) 2 ·6H 2 O changed to 41.3g of Fe 2 (SO 4 ) 3 and 150g of MgSO 4 , 124g of urea was changed to 163.3g of ammonium bicarbonate, and the rest of the conditions remained unchanged. After the reaction product was washed with water and alkali to remove HCl and HF, the conversion rate of 1,1,2,3-tetrachloropropene was 100% and the selectivity of HCFC-1233xf was 99.3% according to gas chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com