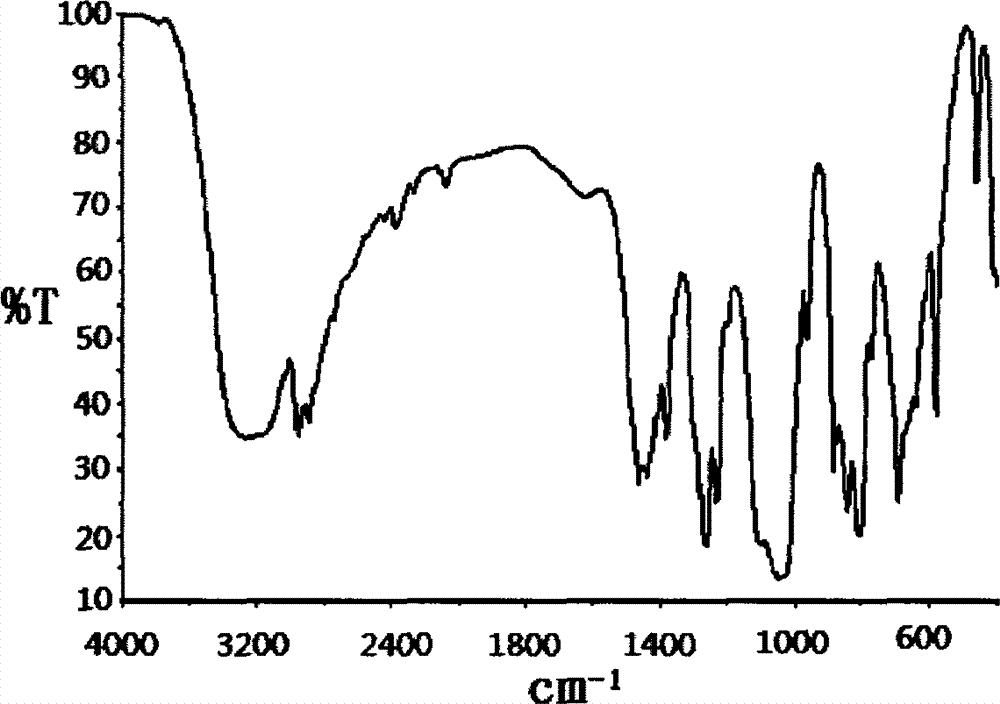
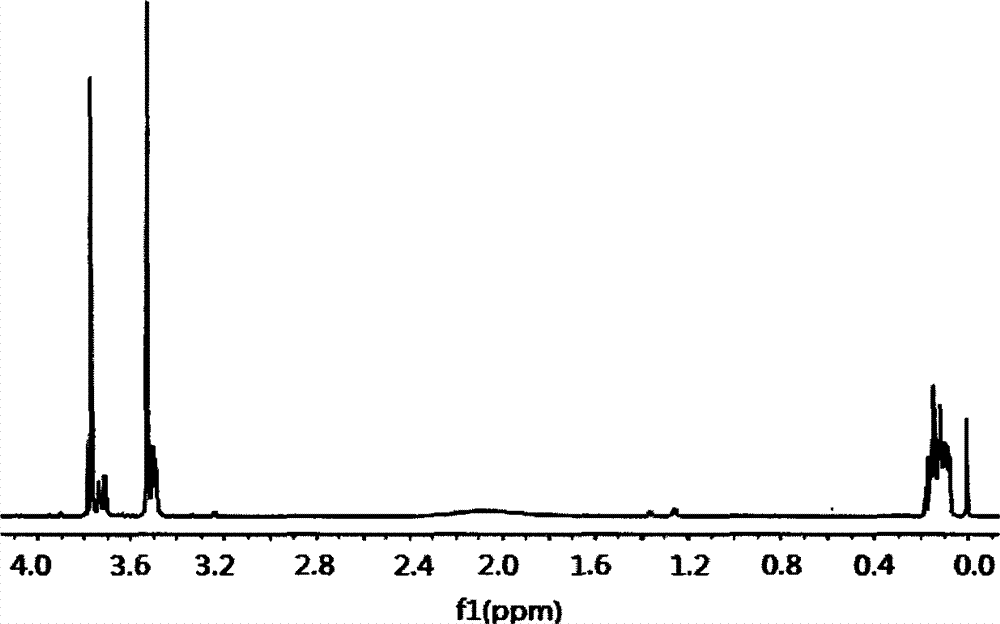
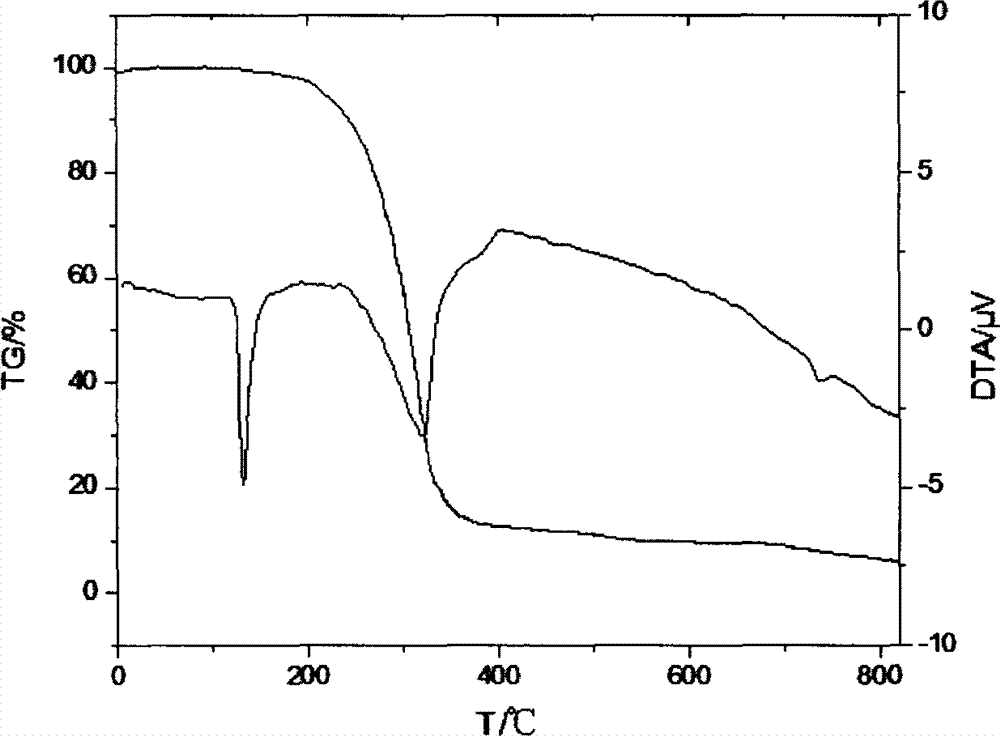
Preparation method of dimethyl dibromo neopentyl dioxy cyclosilane compound as fire retardant
A technology of dimethyldibromoneopentyldioxycyclosilane and dibromoneopentyl glycol, which is applied in the field of preparation of flame retardant dimethyldibromoneopentyldioxycyclosilane compounds, and can solve the problem of halogenated The development of flame retardants is restricted and other issues, and the effect of cheap raw materials, low cost and good compatibility is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 In a 100ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency fractionation device, 13.1g (0.05mol) of dibromoneopentyl glycol, 6.01g (0.05mol) of dimethyldimethoxysilane and 40ml of toluene, heated up to 80°C to start the boiling fractionation reaction, controlled the top temperature of the fractionation column not higher than 65°C, and continuously separated the generated methanol. As the reaction progressed, the free dimethyldimethoxysilane decreased, and the reaction system Gradually raise the temperature, and keep it warm at 110°C for about 7 hours. When the fractionated methanol reaches the theoretical amount, stop the reaction, distill off the organic solvent (recycled for use) and a small amount of low boiling point substances, and add the product mass in grams twice the volume in milliliters of water, stirred for 30 min, filtered with suction, rinsed with water of 0.5 times the volume in milliliters of the product mass in g...
Embodiment 2
[0024] Example 2 In a 100ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency fractionation device, 13.1g (0.05mol) of dibromoneopentyl glycol, 6.01g (0.05mol) of dimethyldimethoxysilane and 50ml of xylene, heated up to 80°C to start the boiling fractionation reaction, controlled the top temperature of the fractionation column not higher than 65°C, continuously separated the generated methanol, and the free dimethyldimethoxysilane decreased as the reaction progressed, and the reaction system Gradually raise the temperature, and keep it warm at 140°C for about 6 hours. When the fractionated methanol reaches the theoretical amount, stop the reaction, distill off the organic solvent (recovered for use) and a small amount of low boiling point substances, and add 2 times the volume of the product in milliliters several times of water, stirred for 30min, suction filtered, then rinsed with water of 0.5 times the volume in milliliters of the product mass i...
Embodiment 3
[0025] Example 3 In a 100ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency fractionation device, 13.1g (0.05mol) of dibromoneopentyl glycol, 6.01g (0.05mol) of dimethyldimethoxysilane and 50ml tetrachloroethane, heat up to 80°C to start the boiling fractionation reaction, control the fractionation column top temperature not higher than 65°C, continuously separate the generated methanol, and the free dimethyldimethoxysilane will decrease as the reaction progresses, The temperature of the reaction system is gradually raised, and the reaction is carried out at 130°C for about 7 hours. When the fractionated methanol reaches the theoretical amount, the reaction is stopped, and the organic solvent (recycled) and a small amount of low boiling point substances are distilled off. Water in milliliters by volume, stirred for 30 minutes, filtered with suction, then rinsed with water of 0.5 times the mass of the product in milliliters by volume, drained, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com