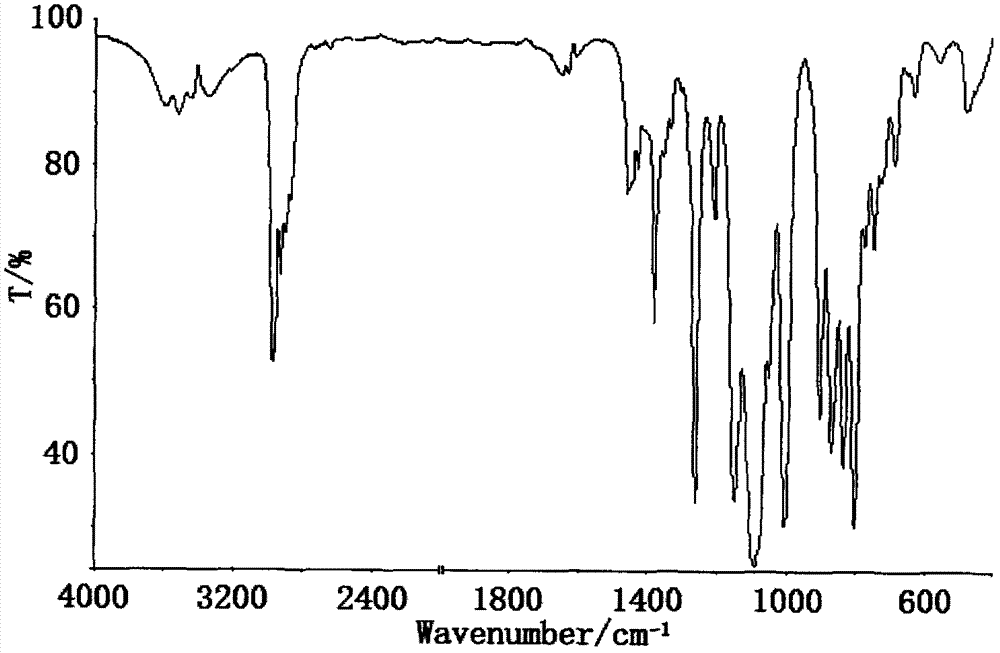
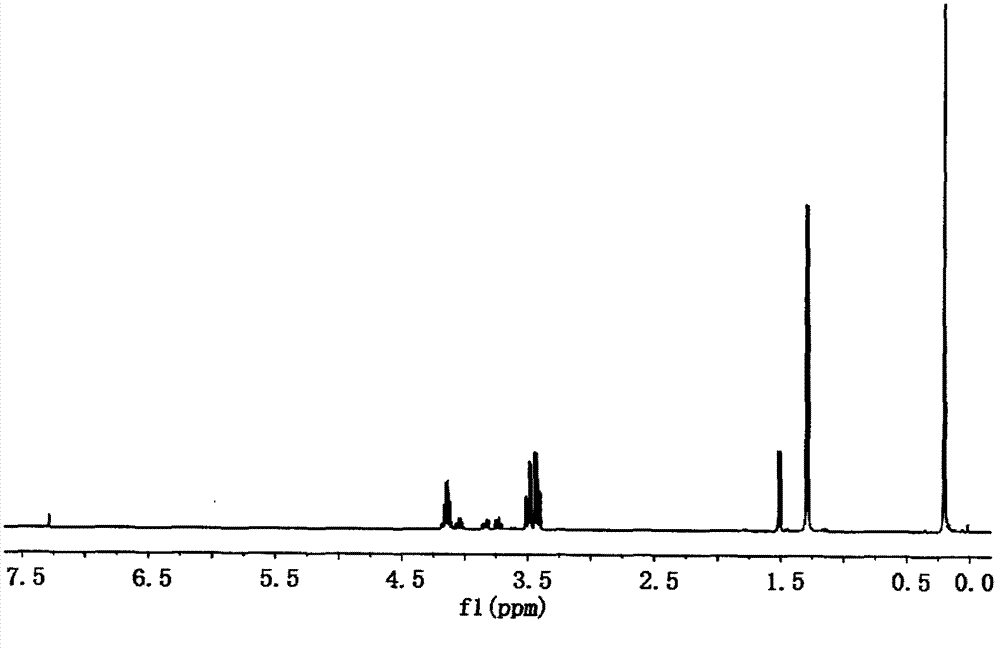
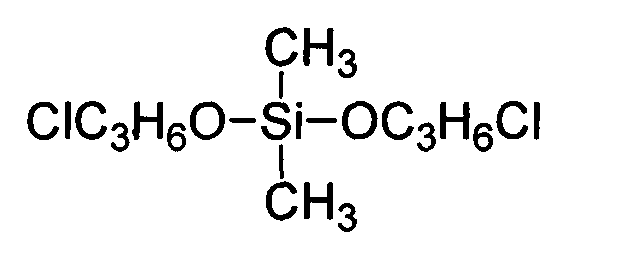Preparation method of dimethyl silicic acid (chlorine acrylate) diester as flame resistant plastifier
A technology of dimethyl silicate and flame retardant plasticizer, which is applied in the field of flame retardant plasticizers, can solve problems such as difficulty in finding substitutes, and achieve good symmetry, good compatibility, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 In a 250ml four-necked bottle equipped with a stirrer, a thermometer and a condenser tube, and a drying tube and a hydrogen chloride absorption device at the top of the condenser tube, replace the air in the bottle with nitrogen, and add 9.45g (0.1mol ) chloropropanol, 65ml of dioxane, add 6.45g (0.05mol) of dimethyldichlorosilane dropwise, control the reaction temperature not higher than 20°C at the rate of addition, raise the temperature to 50°C after dropping, and keep the reaction for 7h; After the HCl gas is released, add 0.12 g of melamine to adjust the pH of the system to 5-6, filter with suction, and distill the filtrate under reduced pressure to remove the solvent and a small amount of low-boiling matter to obtain di(monochloropropyl) dimethyl silicate. Yield 90.8%.
Embodiment 2
[0035] Example 2 In a 250ml four-necked bottle equipped with a stirrer, a thermometer and a condenser tube, and a drying tube and a hydrogen chloride absorption device at the top of the condenser tube, replace the air in the bottle with nitrogen, and add 10.40g (0.11mol ) Chloropropanol, 50ml acetonitrile, add 6.45g (0.05mol) dimethyldichlorosilane dropwise, control the reaction temperature not higher than 20°C with the drop rate, raise the temperature to 40°C after the drop, keep the temperature for 9h; wait for HCl gas After the release, add 0.25g melamine to adjust the pH of the system to 5-6, filter with suction, and distill the filtrate under reduced pressure to remove the solvent, excess chloropropanol (recycled for use) and a small amount of low boiling point substances to obtain dimethicic acid dimethicone (Monochloropropyl) ester, yield 91.4%.
Embodiment 3
[0036]Example 3 In a 250ml four-necked bottle equipped with a stirrer, a thermometer and a condenser tube, and a drying tube and a hydrogen chloride absorption device at the top of the condenser tube, replace the air in the bottle with nitrogen, and add 11.82g (0.125mol ) Chloropropanol, 45ml ethylene glycol dimethyl ether, add 6.45g (0.05mol) dimethyldichlorosilane dropwise, control the reaction temperature not higher than 20°C with the dropping rate, raise the temperature to 70°C after dropping, and keep warm for reaction 5h; after the HCl gas is released, add 0.37g of melamine to adjust the pH of the system to 5-6, filter with suction, distill the filtrate under reduced pressure to remove the solvent, excess chloropropanol (recycled for use) and a small amount of low boiling point substances to obtain two Bis(monochloropropyl) methyl silicate, yield 93.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com