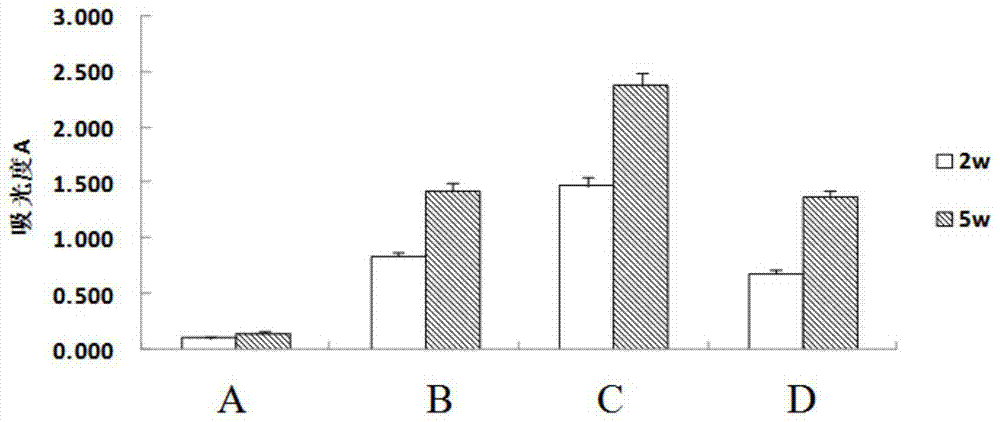
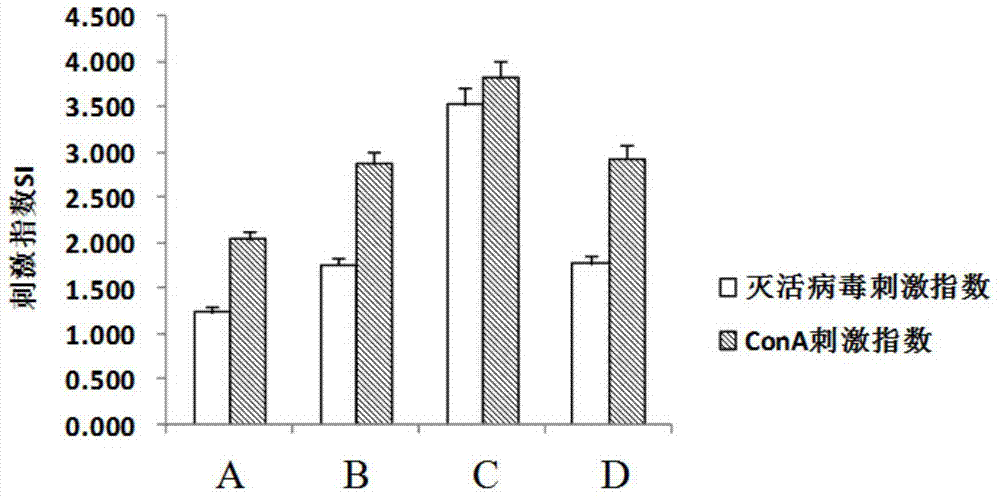
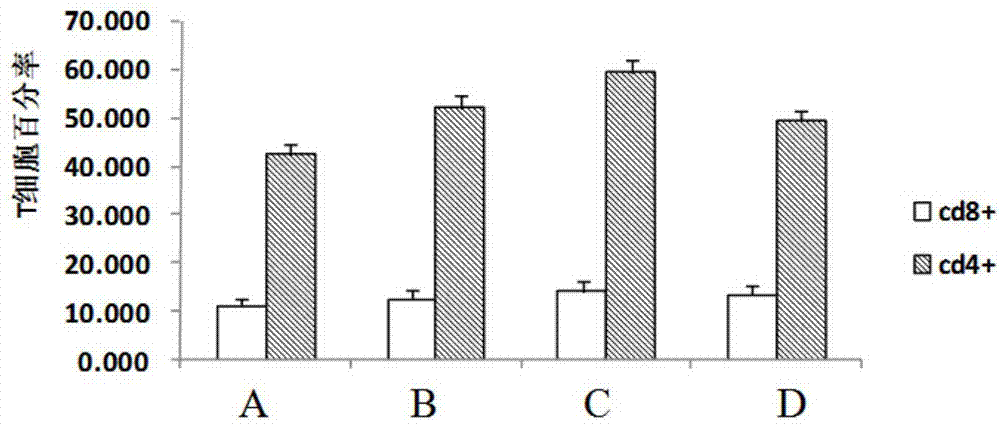
Vaccine for preventing herpesvirus hominis type II
A herpes simplex virus, vaccine technology, applied in antiviral agents, medical preparations containing active ingredients, gene therapy and other directions, can solve the problems of affecting practical application, low efficiency, etc. The effect of morbidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the acquisition of eukaryotic expression plasmid
[0017] The eukaryotic expression plasmid vector pcDNA3-Kan was constructed by replacing the original ampicillin resistance (Amp) on the vector pcDNA3 with Kanna resistance (Kan) by molecular biology techniques PCR, enzyme digestion, ligation and clone screening. The complete gene sequence (gD) of glycoprotein D of HSV-2 sav strain was amplified by PCR method, and the recombinant plasmid pcDNA-Kan / gD containing gD was obtained after cloning and recombination. The recombinant plasmid pcDNA-Kan / gD and its specific preparation method have been compared with "He Fang et al. Chinese Journal of Health Inspection, 2008, 18(6): 997-999".
Embodiment 2
[0018] Embodiment 2: the acquisition of recombinant plasmid
[0019] The activated T cells were taken, the total RNA was extracted with RNeasy Mini Kit (QIAGEN), and the first-strand cDNA was synthesized by reverse transcription with Oligod(T)n as a primer. Referring to the sequence number BC119225.1 included in the NCBI nucleic acid database, a pair of primers were designed, and the synthesized cDNA was used as a template to amplify to obtain SEQ ID NO.1. The upstream primer sequence is 5 , -GCC GAATTC ATGATAGAAACATACAGCCAACCT-3 , , the downstream primer sequence is 5 , -GAC CTCGAG TCAGAGTTTGAGTAAGCCAAAAGA-3 , .
[0020] The gene sequence shown in SEQ ID NO.1 and pcDNA3-Kan were digested with EcoRI and Xho I, purified and recovered by gel cutting. The resulting fragments were recovered and ligated with T4 ligase at a molar concentration of 1:5. The ligation product was transformed into a competent strain of DH5α, and the next day, a medium-sized white colony was pic...
Embodiment 3
[0022] Embodiment 3: The plasmid that embodiment 1 and 2 obtain is used for immune mouse
[0023] 1. Experimental materials:
[0024] 1) The plasmids obtained in Examples 1 and 2 were respectively amplified in E.coli and purified with QIAGEN purification kit. The concentration and purity of the plasmid were measured by ultraviolet spectrophotometry. The concentration and purity of the DNA were determined by OD260 and OD280. The plasmid DNA with a ratio of OD260 / OD280 of 1.8-2.0 was selected to immunize mice. The two plasmids were cryopreserved at -20°C, thawed before immunizing mice, and the two plasmids were mixed according to the mass ratio of 2:1.
[0025] 2) Frozen HSV-2 virus sav3 was repeatedly frozen and thawed three times. After centrifugation, the supernatant was added to the monolayer of Vero cells and incubated at 37°C for 1 h. Remove the virus liquid, add 4% FBS DMEM complete medium, and incubate at 35°C for 24-48h. Adherent cells were scraped off with a cell s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com