Method for preparing serum-free cultured suspension mammal cell line, prepared cell line thereof and application thereof
A serum-free medium and mammalian technology, applied in animal cells, vertebrate cells, artificial cell constructs, etc., can solve the problems of increased risk of external contamination, inappropriate production of animal vaccines, unfavorable proliferation of influenza viruses, etc. , to achieve the effect of reducing the risk of external source pollution, saving manpower, ensuring stability and uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1. Adapt to the domestication of single-cell serum-free pure suspension culture MDCK cells
[0031] (1) Resuscitate a MDCK cell adherent culture. The method of cell resuscitation is to quickly dissolve at 37°C and inoculate into MEM medium containing 5% PAA serum. The number of cells in each cryopreservation tube is at least 3 million, and culture in a 37°C incubator for 24 hours, and replace it with a fresh one containing 5% PAA serum. MEM medium of PAA. After 72 hours, the cells grew into a dense monolayer, digested with 0.125% trypsin, and passaged according to the dispersion ratio of 1:3.
[0032] (2) After resuscitation, serially passage 3 times, use VP-SFMAGT with 5% (v / v) PAA added TM (Gibco company) serum-free medium (or MDCK cell serum-free protein-free chemical medium (Jiangyin Cambridge Biotechnology Co., Ltd.), or InVitrus TM (Cell Culture Technologies, Switzerland), or SFM4MegaVir TM (HyClone Company)) was replaced with MEM containing 5% (v / v...
Embodiment 2
[0038] Embodiment 2 The specific morphology of cells under the large-scale culture conditions of the MDCK cell bioreactor adapted to serum-free suspension culture
[0039] 1. Cell culture in a bioreactor: First, directly resuscitate frozen MDCK cells adapted to serum-free suspension culture in shake flasks, change the medium for 24 hours, and culture at a cell density of more than 4 million / ml in about 72 hours. 3 times, the cell growth resumed; continue to expand the culture, inoculate Bioflo115 fermenter, the inoculation density is 500,000 / ml, and the culture conditions are 37°C, 80-110rpm, DO30%-50%, pH7.2. The medium used in this process is VP-SFMAGT TM (Gibco Company), 0.2% Pluronic F-68 and 30 μg / ml dextran sulfate were added.
[0040] 2. During the cultivation process of the bioreactor, sample and count the number of cells at 18h, 24h, 42h, 48h, 66h, 72h, 90h, 96h, and 114h after inoculation, and stop sampling when the cell viability drops below 50%. , see Table 1 for...
Embodiment 3
[0047] Example 3. Serum-free pure suspension culture breeding avian influenza H9 subtype
[0048] 1, bioreactor culture cell, culture method is the same as embodiment 1;
[0049] 2. When the density of the cells in the fermenter reaches the requirements for inoculating the avian influenza virus, inoculate the virus, and add the virus liquid according to the inoculation dose of MOI=0.001-0.1. In addition, because TPCK-trypsin has a great influence on the proliferation of influenza virus, Therefore, add 1.0-5.0 μg / ml after virus inoculation. The culture conditions are 33℃, 80~110rpm, DO30~50%, pH7.20;
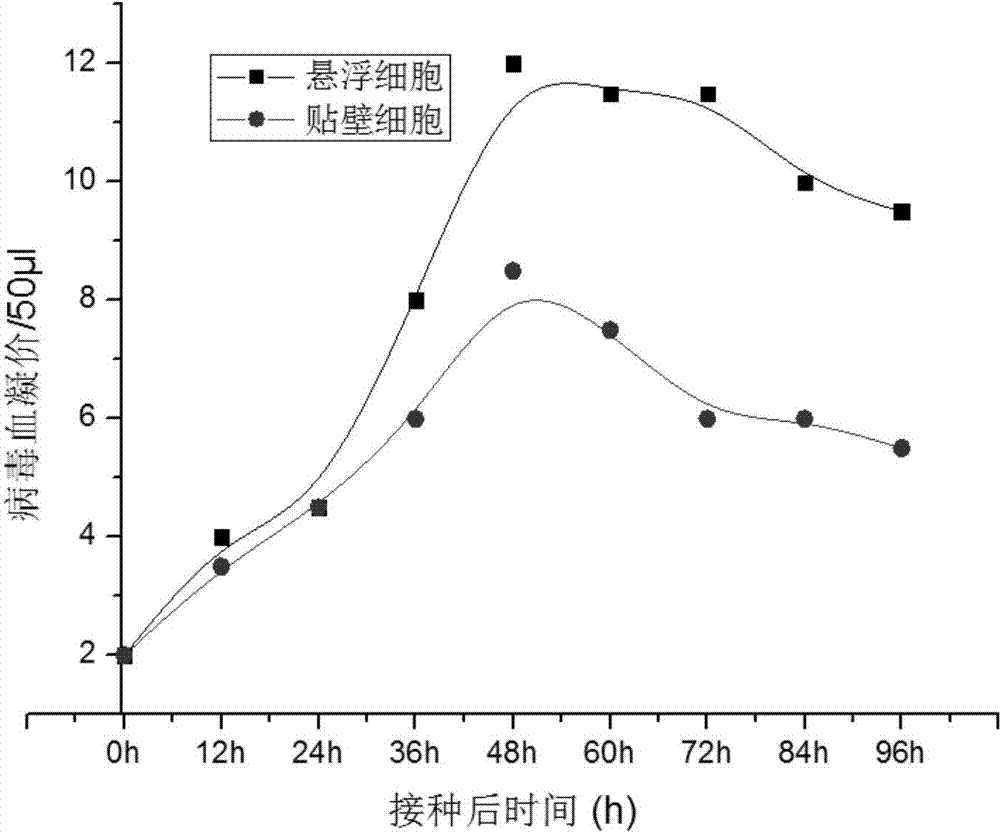
[0050] 3. Since the suspension cells cannot observe cell lesions, samples are taken every 12 hours after inoculation, the number of cells and HA are monitored to determine the virus content, an extreme value of the virus hemagglutination value is found, and the virus liquid is harvested when the virus hemagglutination value is the highest. Cell debris was removed by filtration ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com