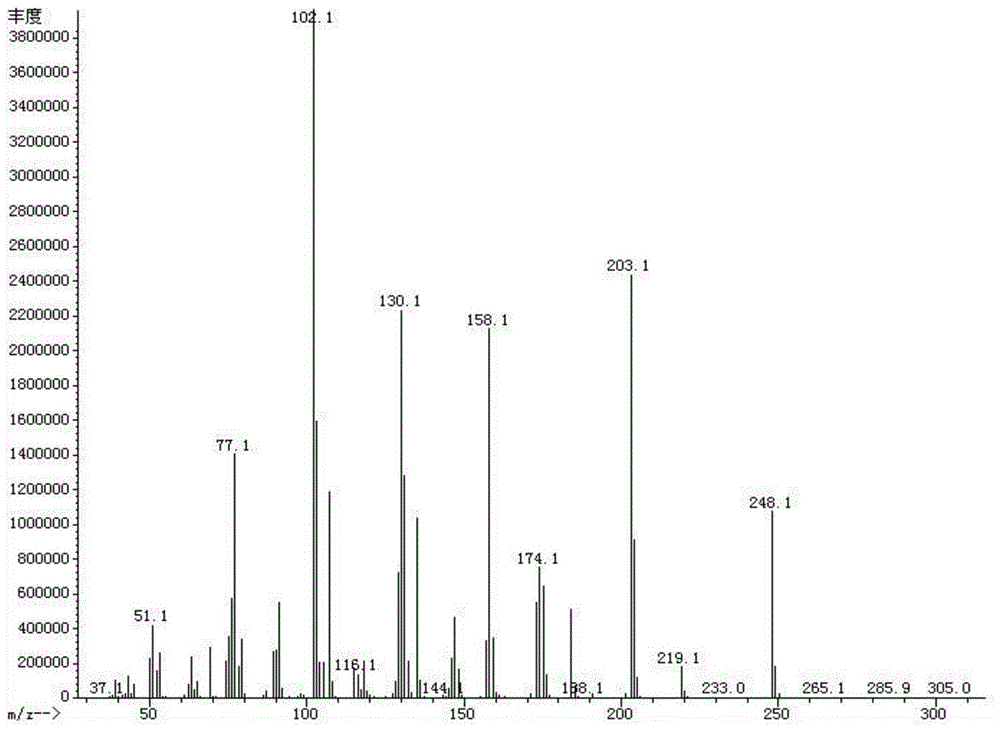
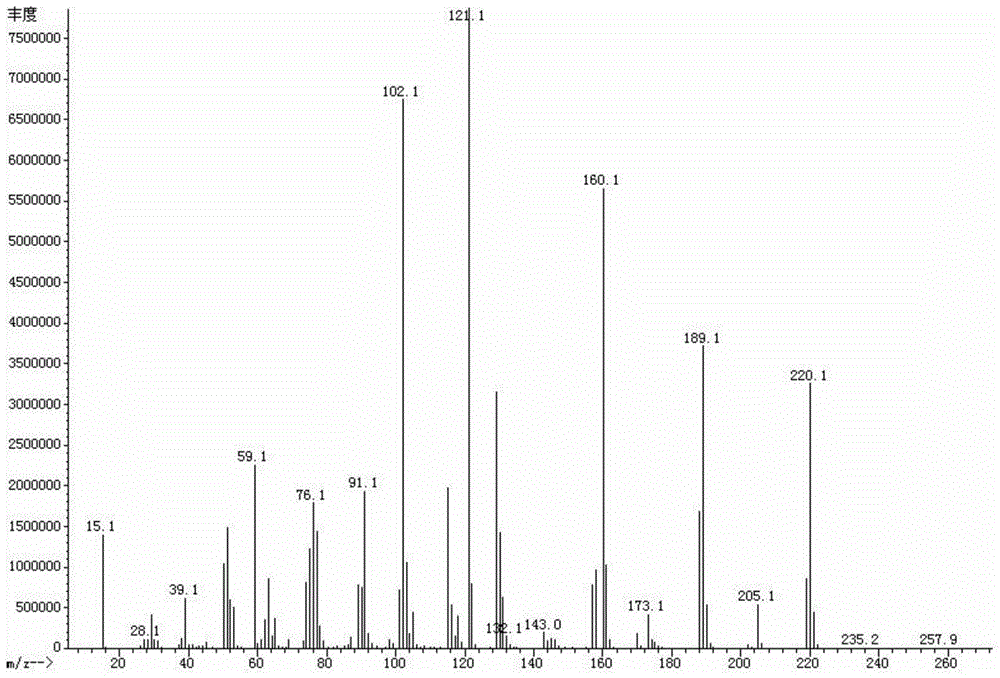
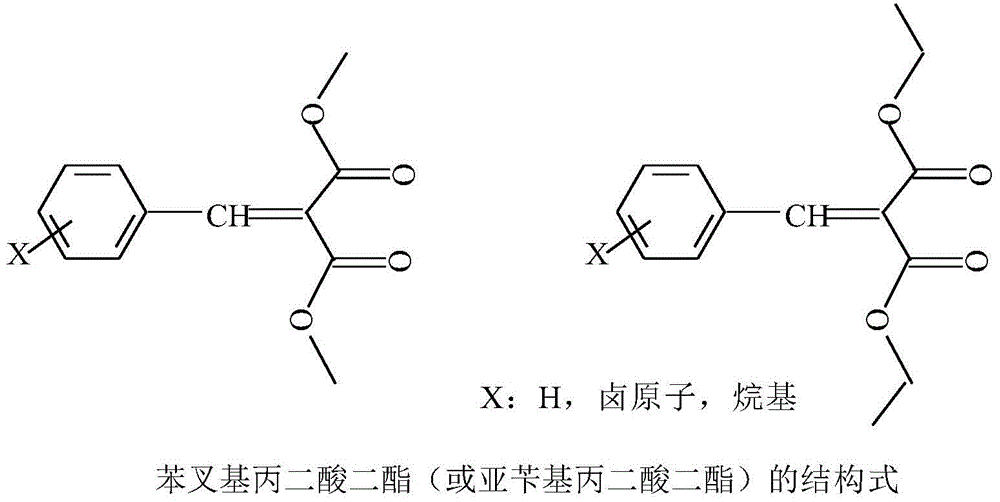
A biomimetic aqueous heterogeneous catalytic system catalyzes the condensation reaction between aromatic aldehydes and malonate diesters
A malonate diester, heterogeneous catalysis technology, applied in the field of fine chemicals of chemical engineering, can solve the problems of useless value by-products, reduced reaction selectivity, loss of raw materials and products, etc., to achieve saving and full utilization, Improve selectivity and yield, reduce the effect of oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 A kind of method that adopts clean technology to synthesize benzoylidene dimethylmalonate comprises the following steps:
[0045] (1) At room temperature, put 50% of the total amount of dimethyl malonate, soluble amino-containing substances, protonic acid, metal salt, and aqueous mixed solvent into the reactor, adjust the pH to 5-6, and , after heating to 30° C., gradually add benzaldehyde or its mixture with the remaining 50% dimethyl malonate. Continue to react at this temperature until the product phase no longer continues to increase;
[0046] (2) From the reaction mixture obtained in step (1), separate out the product-rich phase and the catalyst-containing solvent phase by means of the difference in specific gravity, wherein the solvent phase is recycled;
[0047] (3) The product-rich phase obtained in step (2) is washed with a 1:1 methanol-water solution to remove a small amount of unreacted raw materials to obtain the condensation product dimethyl p...
Embodiment 2
[0048] Embodiment 2 A kind of method that adopts clean technique to synthesize diethyl phenylidene malonate comprises the following steps:
[0049] (1) Put diethyl malonate accounting for 50% of the total amount, amine-containing substances insoluble in solvents, protonic acids, metal salts, and water-containing mixed solvents into the reactor, adjust pH 7 to 8, and simulate sunlight irradiation After heating up to 50°C, gradually add the mixture of benzaldehyde and the remaining 50% diethyl malonate. Continue the reaction at this temperature until the product phase does not continue to increase;
[0050] (2) From the reaction mixture obtained in step (1), use the difference in specific gravity to separate the product-rich phase, solvent phase and solvent-insoluble solid phase containing amine-based substances, and the latter two can be recycled;
[0051] (3) adopt underpressure distillation to the rich product phase that step (2) obtains, to slough a small amount of unreacte...
Embodiment 3
[0052] Embodiment 3 A kind of method that adopts clean technology to synthesize dimethyl benzomalonate comprises the following steps:
[0053] (1) Put all insoluble amine-containing substances, protonic acids, metal salts, aqueous mixed solvents, aromatic aldehydes and dimethyl malonate into the reactor, adjust the pH to 6-7, and heat up to 25 After ℃, maintain this temperature and continue the reaction until the product phase does not continue to increase;
[0054] (2) From the reaction mixture obtained in step (1), use the difference in specific gravity to separate the product-rich phase, the solvent phase and the solid phase amine-containing substance insoluble in the solvent phase, and the latter two can be recycled;
[0055] (3) The product-rich phase obtained in step (2) is washed with 1:1 (volume ratio) methanol-water solution to remove a small amount of unreacted raw materials to obtain the condensation product dimethyl phenylidene malonate or substituted phenylidene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com