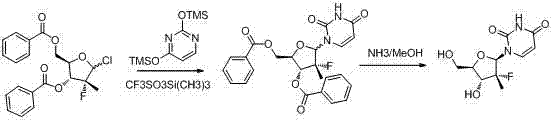
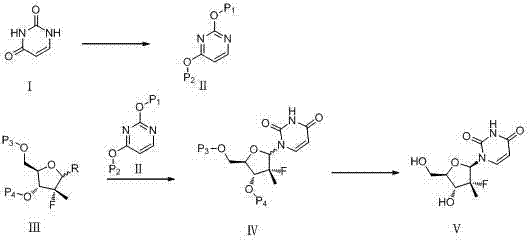
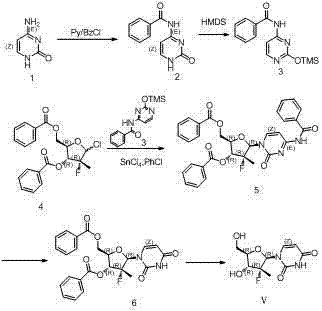
Synthesis method for (2'R)-2'-deoxy-2'-fluorine-2'-methyl uridine
A synthetic method, the technology of methyluridine, which is applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of high process cost, complicated reaction steps, and low overall yield, and achieve saving The effect of solvent consumption, short steps, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Synthesis of 2,4-bis(trimethylsiloxy)pyrimidine: Under argon protection and stirring, add uracil (11.2 g, 0.1mol), ammonium sulfate (1.32 g 0.01mol), HMDS (33.8 g, 0.21mol). The temperature of the oil bath was raised to reflux of chlorobenzene (at this time, the temperature of the oil bath was about 150°C), and the reaction was stirred for 2 hours until the reaction system was dissolved, and then the reaction was continued for half an hour. Post-processing: Cool to room temperature, concentrate with a rotary evaporator (70°C), and then obtain a syrupy substance, which is sealed under argon protection and used directly for the next reaction.
Embodiment 2
[0033]
[0034] Synthesis of (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine:
[0035] Operation: Stir at room temperature and under the protection of argon, add 3R, 4R, 5R-2-chloro-3-fluoro-3-methyl-4-benzoyl-5-benzoic acid methyl tetrahydrofuran ( 3.92 g, 0.01 mol), SnCl 4 (10.4 g, 0.04 mol), 2,4-bis(trimethylsiloxy)pyrimidine (5.12 g, 0.02 mol), chloroform 40mL, the oil bath was heated to chloroform reflux. At this time, the internal temperature is about 63°C. Stirring was maintained at this temperature for 16 hours. Post-treatment: Cool to room temperature, pour the reaction solution into dilute hydrochloric acid, continue to stir for about 30 minutes, filter with suction, and wash the filter cake repeatedly with DCM (repeat this operation 3 to 4 times until TLC judges that there is no product in the filtrate). All filtrates were combined and concentrated under reduced pressure with a rotary evaporator to obtain a dark brown solid oil mixture (LCMS detection). Dissolve th...
Embodiment 3
[0038]
[0039] Synthesis of (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine:
[0040] Operation: Stir at room temperature and under the protection of argon, add 3R, 4R, 5R-2-chloro-3-fluoro-3-methyl-4-benzoyl-5-benzoic acid methyl tetrahydrofuran ( 3.92 g, 0.01 mol), ZnCl 2 (5.44 g, 0.04 mol), 2,4-bis(trimethylsiloxy)pyrimidine (5.12 g, 0.02 mol), chloroform 40mL, the oil bath was heated to chloroform reflux. At this time, the internal temperature is about 63°C. Stirring was maintained at this temperature for 16 hours. Post-treatment: Cool to room temperature, pour the reaction solution into dilute hydrochloric acid, continue to stir for about 30 minutes, filter with suction, and wash the filter cake repeatedly with DCM (repeat this operation 3 to 4 times until TLC judges that there is no product in the filtrate). All filtrates were combined and concentrated under reduced pressure with a rotary evaporator to obtain a dark brown solid oil mixture (LCMS detection). Dissolve th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com