Bovine serum albumin surface molecular imprinted polymer and preparation method thereof
A bovine serum albumin and surface molecular imprinting technology, which is applied in the fields of chemistry and materials, can solve the problems of imprinting research difficulties, large volume, variability and inactivation, etc., and achieve the effect of good reusability, large adsorption capacity, and rapid adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
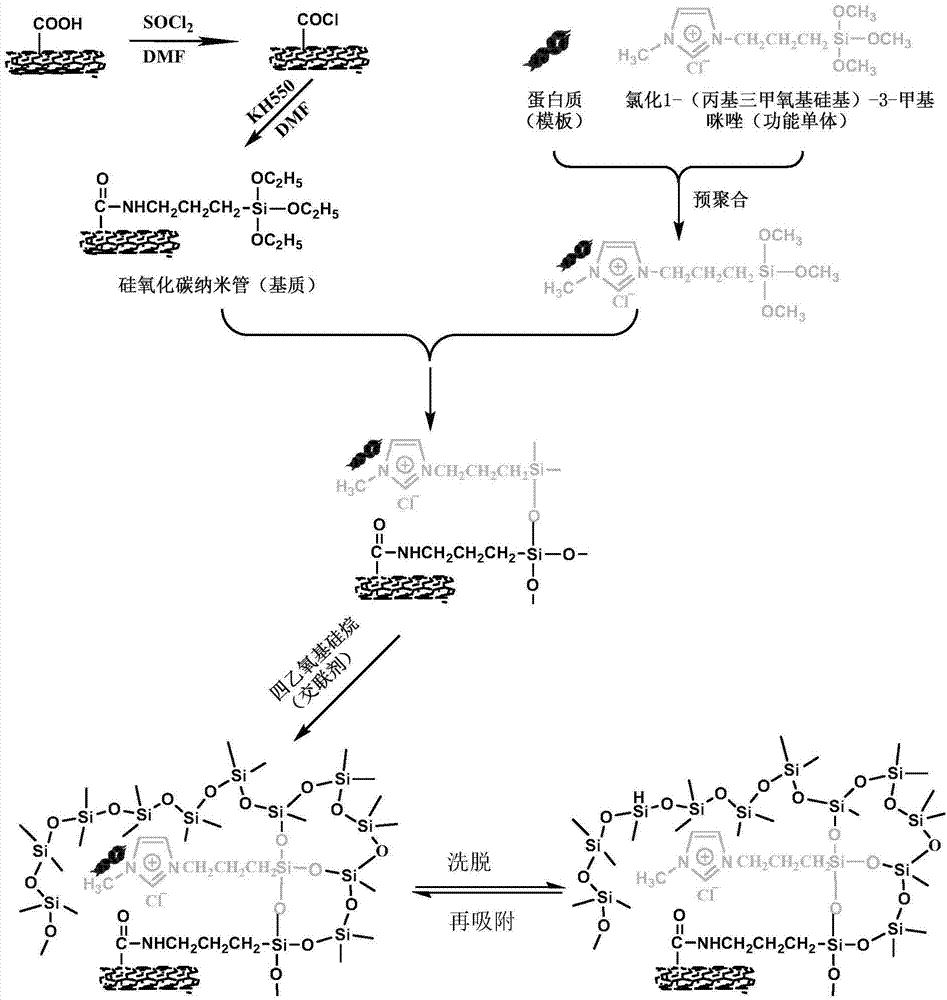
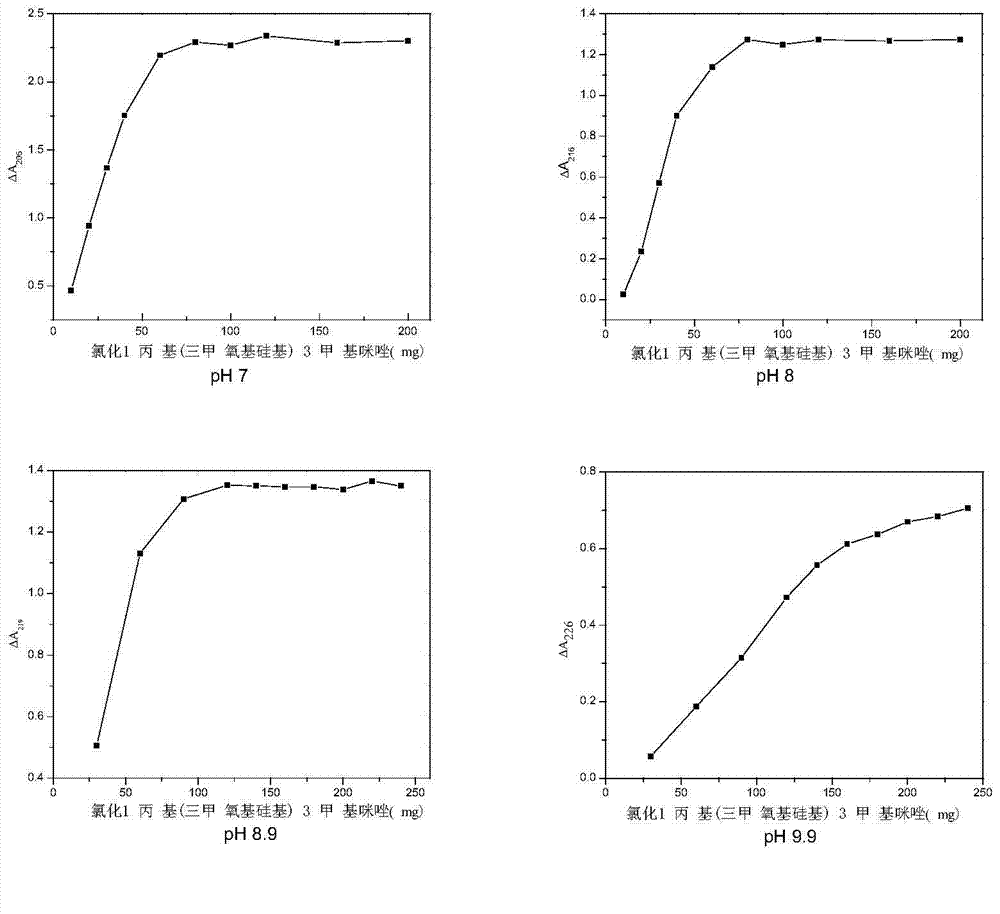
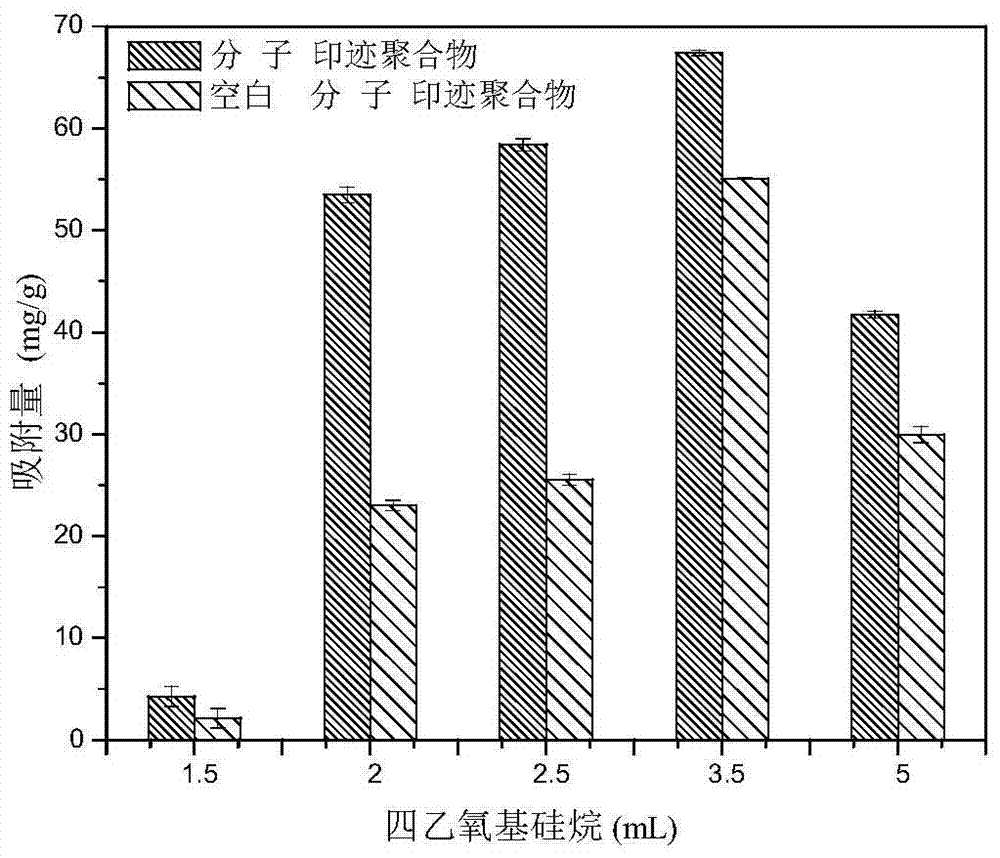
[0035] Add 10 mg of γ-aminopropyltriethoxysilane-modified carbon nanotubes to 10 mL of pH7 phosphate buffer solution, place in a 100 mL Erlenmeyer flask for ultrasonic dispersion for 30 s, and magnetically stir at room temperature for 12 h to obtain the first solution. Dissolve 30mg of bovine serum albumin and 45mg of ionic liquid 1-propyl(trimethoxysilyl)-3-methylimidazole chloride in 10mL of pH7 phosphate buffer solution, and place in a refrigerator at 4°C for 12h to obtain the second solution . Dissolve 2.5 mL of tetraethoxysilane in 12.5 mL of pH7 phosphate buffer, mix the first solution with the second solution, stir magnetically at 4°C for 12 hours, then add tetraethoxysilane solution to the mixed solution, Magnetic stirring was continued at 4 °C for 48 h. After the reaction is completed, the solid and liquid phases are separated, and the solid phase product is washed four times with a phosphate buffer solution of pH 7, then eluted with ultrapure water until no protein ...
Embodiment 2
[0037]Add 10 mg of γ-aminopropyltriethoxysilane-modified carbon nanotubes to 10 mL of Tris-HCl buffer solution at pH 8, place in a 100 mL Erlenmeyer flask for ultrasonic dispersion for 30 s, and magnetically stir at room temperature for 12 h to obtain the first solution. Dissolve 30 mg of bovine serum albumin and 60 mg of ionic liquid 1-propyl (trimethoxysilyl)-3-methylimidazole chloride in 10 mL of Tris-HCl buffer solution at pH 8, and place in a refrigerator at 4°C for 12 hours to obtain the second parts solution. Dissolve 2.5 mL of tetraethoxysilane in 12.5 mL of Tris-HCl buffer solution with pH 8, mix the first solution with the second solution, stir magnetically at 4°C for 12 hours, then add tetraethoxysilane to the mixed solution The solution was continued to be magnetically stirred at 4°C for 48h. After the reaction is completed, the solid-liquid phases are separated, and the solid-phase product is washed four times with a Tris-HCl buffer solution with a pH of 8, and t...
Embodiment 3
[0039] Add 10 mg of γ-aminopropyltriethoxysilane-modified carbon nanotubes to 10 mL of Tris-HCl buffer solution with pH 8.9, place in a 100 mL Erlenmeyer flask for ultrasonic dispersion for 30 s, and magnetically stir at room temperature for 12 h to obtain the first solution . Dissolve 30 mg of bovine serum albumin and 80 mg of ionic liquid 1-propyl(trimethoxysilyl)-3-methylimidazole chloride in 10 mL of Tris-HCl buffer solution at pH 8.9, and place in a refrigerator at 4°C for 12 hours to obtain the first Two solutions. Dissolve 2.5 mL of tetraethoxysilane in 12.5 mL of Tris-HCl buffer solution with pH 8.9, mix the first solution with the second solution, stir magnetically at 4°C for 12 hours, and then add tetraethoxysilane to the mixed solution Base silane solution, continue to magnetically stir at 4°C for 48h. After the reaction is completed, the solid-liquid phases are separated, and the solid-phase product is washed four times with a Tris-HCl buffer solution with a pH o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com