Riemerella anatipestifer lipopolysaccharide mutant strain and application thereof
A technique for Riemer's duck disease and lipopolysaccharide, which is applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1R
[0036] The construction of embodiment 1RA CH3 random mutation library
[0037] (1) Transposon random mutation method to construct RA CH3 random mutation library
[0038] According to the method reported in the literature[9] E.coli BW19851 (contains transposon plasmid Tn4351) and RA strain CH3 (kanamycin resistance) were cultured separately, followed by conjugative transduction according to the ratio of donor bacteria:recipient bacteria at 1:2, and cultured at 30°C for 8 hours Spread on TSB plates containing erythromycin and kanamycin, 37°C, 5% CO 2 Continue to cultivate in the incubator for 24-30h. Pick a single colony into TSB containing double antibody, shake at 37°C until it grows. Use the primers Erm F / R (Table 1) designed by the erythromycin gene and the 16S rRNA primers 16S rRNA F / 16S rRNA R (Table 1) of RA to identify the mutant strains by PCR. Double positives are positive bacteria, and a random mutation library is constructed .
Embodiment 2
[0039] The preparation of embodiment 2RA LPS monoclonal antibody
[0040] proceed as usual [10] . The RA CH3 strain was cultivated to the logarithmic phase, and 0.4% formalin was added to inactivate it for 18 hours. The inactivated bacterial solution was emulsified with an equal volume of Freund's complete adjuvant (Sigma-Aldric), and the volume was 1×10 8 Dose of CFU / mouse BALB / c female mice (6-8 weeks old, provided by Shanghai Slack Experimental Animal Co., Ltd.) were subcutaneously injected into the back and abdomen at multiple points for the first immunization. Immunization was boosted three times every two weeks with Freund's incomplete adjuvant (Sigma-Aldrich) plus an equal volume of inactivated CH3 bacterial fluid emulsification, and the dose, method, and route were the same as the first immunization. 7 days after the last immunization, the serum antibody titer was detected, and CH3 bacterial solution (1×10 8 CFU / only, no adjuvant added), after 3 days, take spleen c...
Embodiment 3
[0041] Identification of embodiment 3 mutant strain RA-M1
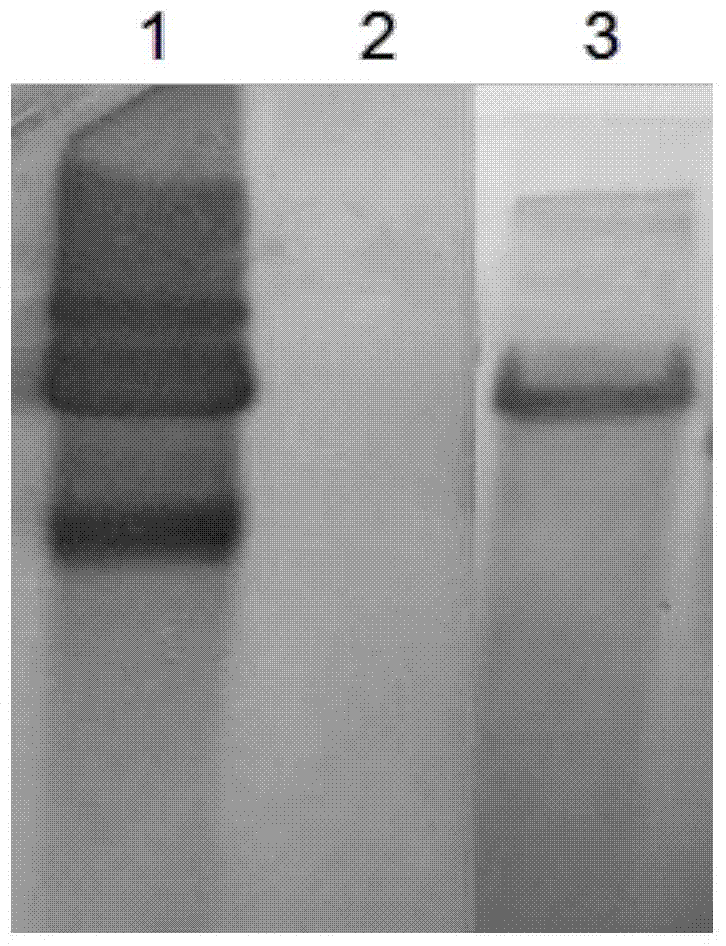
[0042] The CH3 random mutant strain was coated with an ELISA plate, and 8A9 ascites monoclonal antibody was used for indirect ELISA screening to obtain a 8A9 monoclonal antibody negative reaction strain (RA-M1). Southern blotting identified transposon Tn4351 inserted into the whole CH3 genome as a single point (Such as figure 1 , showing only a single Tn4351 insertion site in the RA-M1 genome).
[0043] Then Genome walking was used to identify the mutation site, as shown in Figure 2a-2c, Figure 2a is the identification map of the first round of PCR products, SP1 was PCRed with AP1-AP4 and the mutant strain respectively; Figure 2b is the second round of PCR products Identification diagram, SP2 was subjected to PCR with AP1-AP4 and the mutant strain, respectively; Figure 2c is the identification diagram of the third round of PCR products, and SP3 was subjected to PCR with AP1-AP4 and the mutant strain, respectively. I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com