Triplet fluorescence quantitative detection kit for PRRSV (Porcine reproductive and respiratory syndrome virus), HP-PRRSV (Highly pathogenic porcine reproductive and respiratory syndrome virus) and CSFV (Classical Swine Fever Virus)
A technology of porcine PRRS virus and detection kit, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, microorganisms, etc., can solve the problems of no simultaneous detection, reduce detection costs, save detection time, and have high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
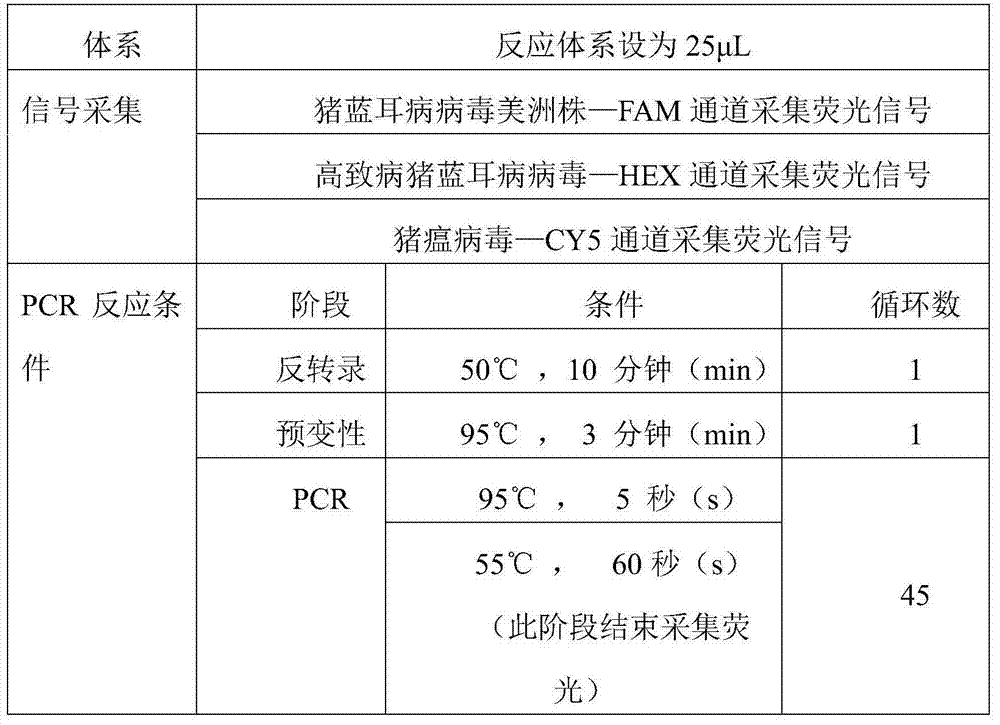
Image
Examples
Embodiment 1
[0035] Example 1 Design and Screening of Primers and Probes
[0036] In RT-PCR detection, the design of primers and probes directly affects the specificity and sensitivity of detection. Especially in multiple RT-PCR detection, there is mutual influence between different primers and probes. After fully considering relevant factors, the present invention designed several sets of primers and probes, but they were not satisfactory. However, it was unexpectedly found that the following sets of primers and probes showed good specificity and sensitivity.
[0037] 1. Specific primers for CSFV
[0038] Upstream primer:TTCAATTTGGTTCAGGGCCTCC
[0039] Downstream primer: TACTCAGGACTTAGACCACCCAGG
[0040] Specific probes for classical swine fever virus
[0041] Fluorescent probe: CY5-ATGCCCATAGTAGGACTAGCAAACGG-BHQ3
[0042] 2. Specific primers for the American strain of PRRS virus
[0043] Upstream primer: TGGTTTCTCTCTGGCTTTTAGGTC
[0044] Downstream primer: GTAGGTTCCATCTGGTGCGGT
...
Embodiment 2
[0053] Example 2 kit assembly
[0054] According to the physical and chemical properties of the reagents, the kit is divided into two parts A and B for assembly. Part A is the virus total RNA extraction reagent, and part B is the fluorescent quantitative PCR detection reagent, which is convenient for storage and transportation.
[0055] Pack the following reagents in suitable outer boxes and label them (name, batch number, production date, expiry date, etc.).
[0056] Part A
[0057] (1) One bottle of solution A (TRIzoL), 25mL;
[0058] (2) One bottle of solution B (chloroform / isoamyl alcohol), 6mL;
[0059] (3) One bottle of solution C (isopropanol), 18mL;
[0060] Part B
[0061] (4) Solution D (RNase Free dH 2 O) two, 2mL;
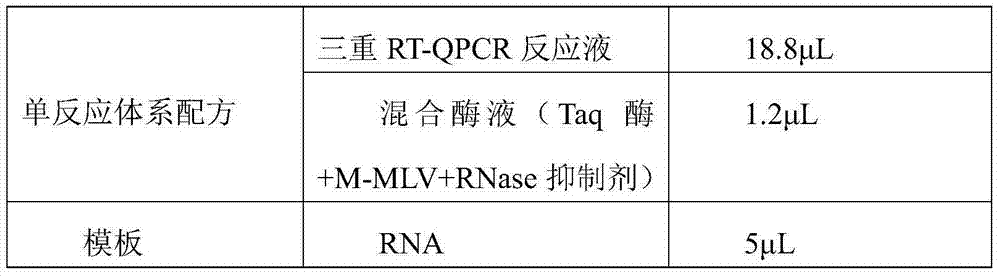
[0062] (5) One solution E (triple RT-QPCR reaction solution), 1.5mL;
[0063] (6) One solution F (mixed enzyme solution), 65 μL;
[0064] (7) One bottle of solution G (positive control), 40 μL;
[0065] (8) One bottle of solution H (negative c...
Embodiment 3
[0089] Embodiment 3 specificity experiment
[0090] 1. Reagents and materials
[0091] 1.1 Reagent The kit of Example 2 was used.
[0092] 1.2 Test materials
[0093] 1.2.1 Common related samples: porcine healthy somatic cell culture, porcine circovirus, pseudorabies virus, swine influenza virus, porcine parvovirus, porcine rotavirus, porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus .
[0094] 1.2.2 Positive samples: tissue and cell culture of porcine PRRS virus (American strain), tissue and cell culture of highly pathogenic porcine PRRS virus, tissue and cell culture of swine fever virus, and the first three mixture.
[0095] Ten copies of each of the above test materials.
[0096] 2. Test method
[0097] Reagent preparation: with absolute ethanol (75mL, add 25mL RNAse Free dH 2 O,) is configured into 75% ethanol, pre-cooled before use.
[0098] 2.1 Total RNA extraction
[0099]2.1.1 Take 100 μL of tissue sample grinding supernatant and pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com