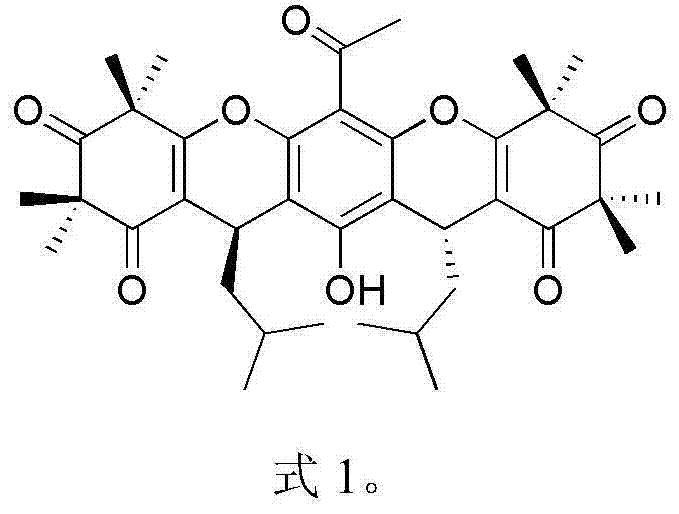
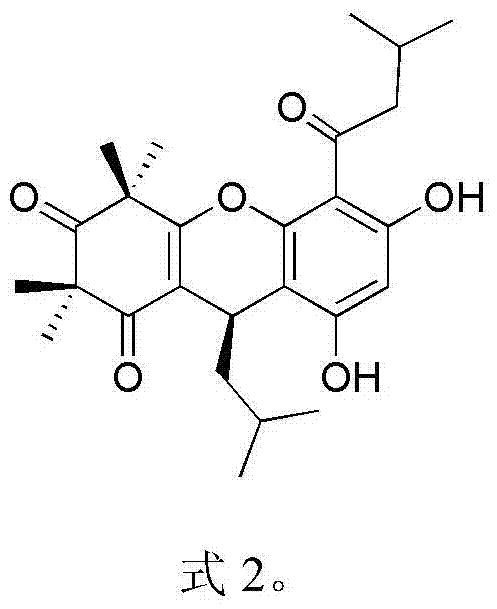
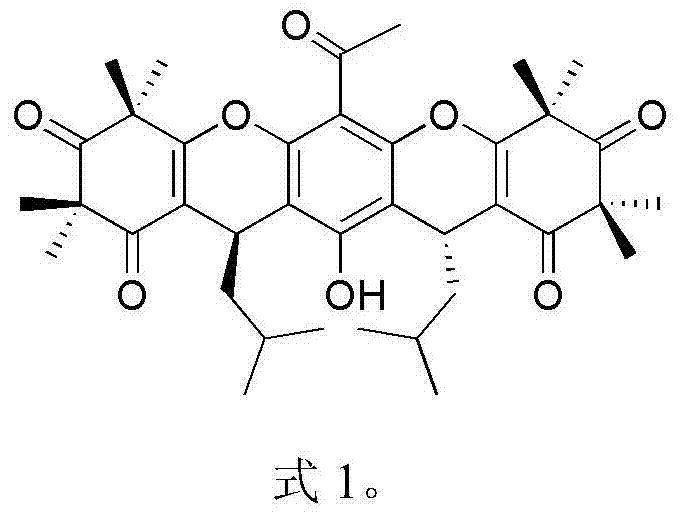
Myrtle ketone compound and application thereof in preparation of antibacterial medicines
An antibacterial drug and compound technology, applied in the field of phytochemistry, can solve the problems of unclear active ingredients and mechanism of action, small side effects, etc., and achieve the effect of good antibacterial activity and strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the separation and purification of compound rhodomyrtosone B and tomentosone C
[0019] The organic solvents used in the separation and extraction, such as chloroform, ethyl acetate, methanol, ethanol, acetone, n-hexane, dichloromethane, etc., have been re-distilled, and other organic solvents used in small amounts are analytically pure; the deuterated reagent is Qingdao Tenglong The product of Microwave Technology Co., Ltd.; the medium pressure preparative chromatography (Dr Flash II) was produced by Shanghai Lisui Chemical Technology Co., Ltd., and the HPLC liquid chromatograph was produced by Tianjin Lanbo Experimental Instrument Equipment Co., Ltd.
[0020] 13 kg of dried myrtle (Rhodomyrtus tomentosa) leaves were pulverized, extracted 3 times with a volume fraction of 95% ethanol aqueous solution, each time for 5 days, and the extracts were combined and concentrated under reduced pressure to obtain 1.8 kg of extract; alkane, ethyl acetate extracted th...
Embodiment 2
[0032] Embodiment 2: the antibacterial activity evaluation of compound
[0033] In this embodiment, the minimum inhibitory concentration (MIC) of the anti-MRSA activity of the sample will be determined by the resazurin chromogenic method. In the test, the 96-well plate dilution titer technique will be used to simultaneously determine the minimum inhibitory concentration (MIC) of various substances.
[0034] First, mix 7.5 mL of indicator solution (100 μg / mL resazurin aqueous solution) with 5 mL of the bacteria solution to be tested (10 8 CFU / mL) and mix well, and add 100 μL of mixed bacterial solution to all test wells in columns 1 to 8. Then add 100 μL of the DMSO solution (16-500 μg / mL) of the sample to be tested into each plate hole in the first column in turn, and after mixing evenly, take out 100 μL of the solution and transfer it to the corresponding plate hole in the second column, and use the same method Doubling dilutions to column 8. Finally, put the orifice plate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com