Medicine for treating irritable bowel syndrome
A technology for irritable bowel syndrome and irritable bowel syndrome, which is applied in the field of drugs for the treatment of irritable bowel syndrome, and can solve problems such as unsuitability for large-scale industrial production and unstable components of crude extracts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This embodiment prepares a kind of medicine for the treatment of irritable bowel syndrome, and the active ingredient of the medicine is total lactones of inulin (content>70%), wherein inulin and iso-inulin account for more than 90% of total lactones .
[0036] The above active ingredients of the medicine are prepared into oral preparations; the oral preparations are enteric-coated tablets, enteric-coated capsules or enteric-coated pellets.
[0037] Above-mentioned medicine preparation method comprises the following steps:
[0038] 1) firstly dry the dried roots of Inula chinense, crush them into coarse powder and soak them in ethanol solution;
[0039] 2) Heating the soaked medicinal material to reflux and recovering the solvent to obtain the inulin extract;
[0040] 3) Carry out silica gel column chromatography and collect the eluate from the inulin extract;
[0041] 4) collecting the solvent in the silica gel column chromatography, and drying the solute under reduced...
Embodiment 2
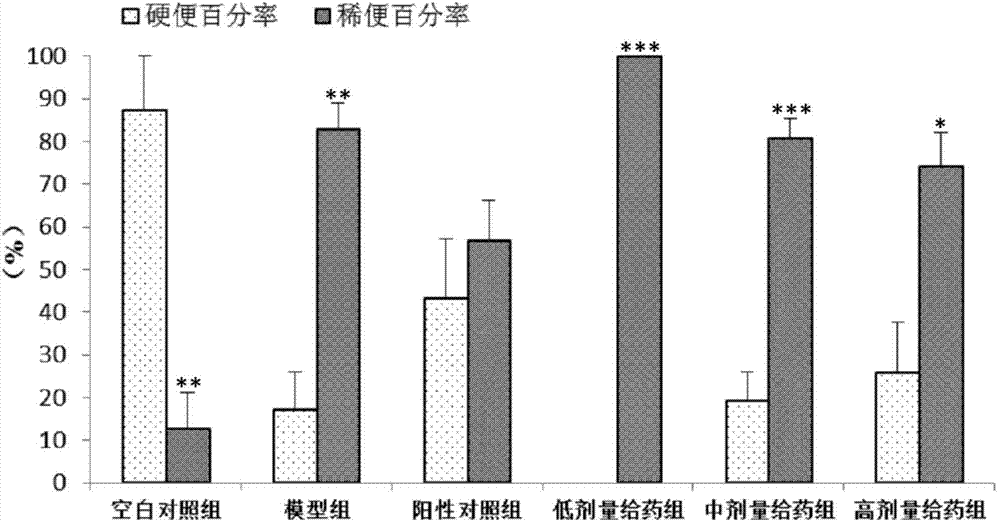
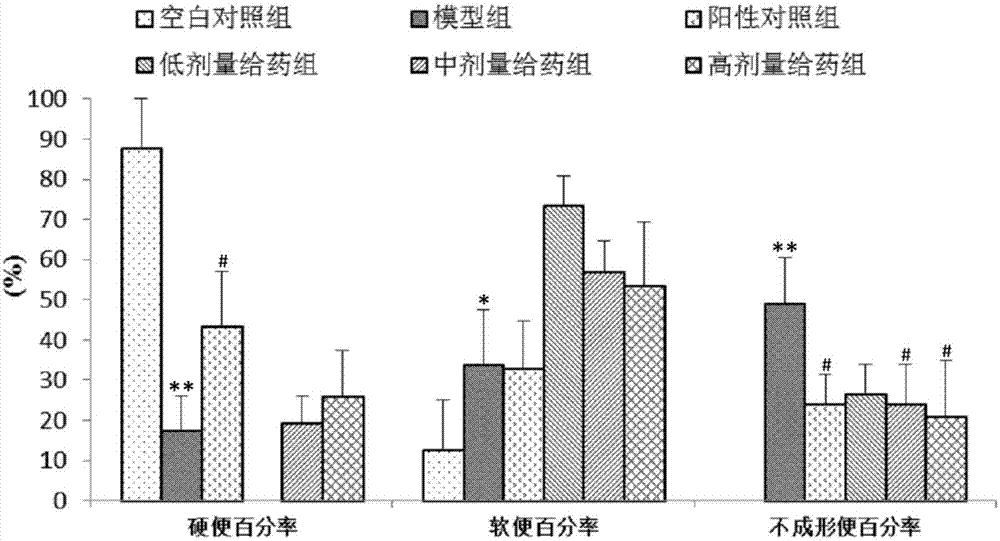
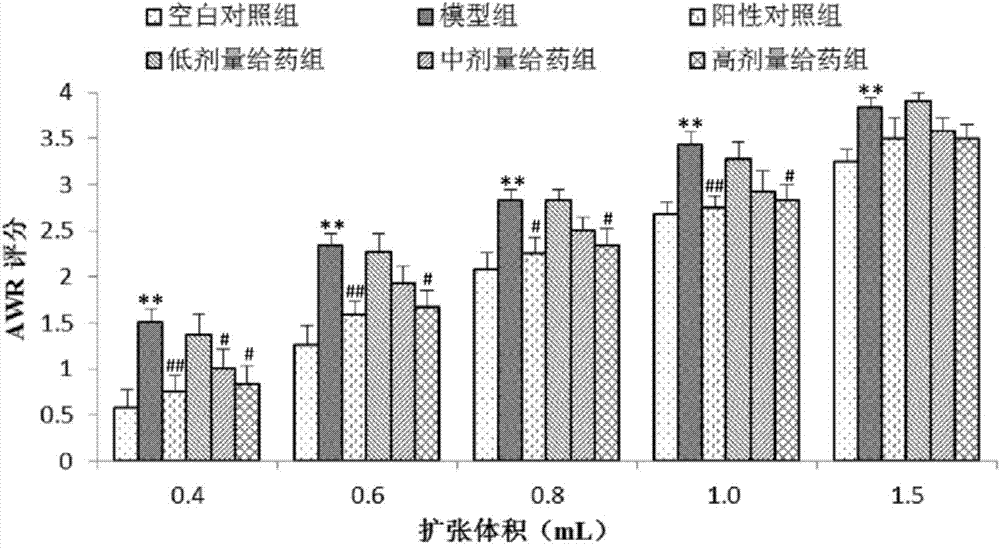
[0065] In this example, the same method as in Example 1 was used to prepare different contents of the medicine for treating irritable bowel syndrome, and its therapeutic effect on rats with constipation-type irritable bowel syndrome was detected, as follows:
[0066] Test animals: male Wistar rats, weighing 120-150 g.
[0067] Test drug:
[0068] Test group 1: 1.8mg / mL pinaverium bromide suspension (positive drug)
[0069] Test group 2: 1mg / mL inulin total lactone emulsion (low dose inulin)
[0070] Test group 3: 3 mg / mL inulin total lactone emulsion (medium dose inulin)
[0071] Test group 4: 9 mg / mL inulin total lactone emulsion (high dose inulin)
[0072] Test equipment: electronic analytical balance; electronic weighing scale; in-cut high-speed homogenizer; ultraviolet-visible spectrophotometer; sinks; ultrasonic cleaners.
[0073] Test method: Rats were randomly divided into groups, and the rats in each group were raised separately in separate cages. In order to elim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com