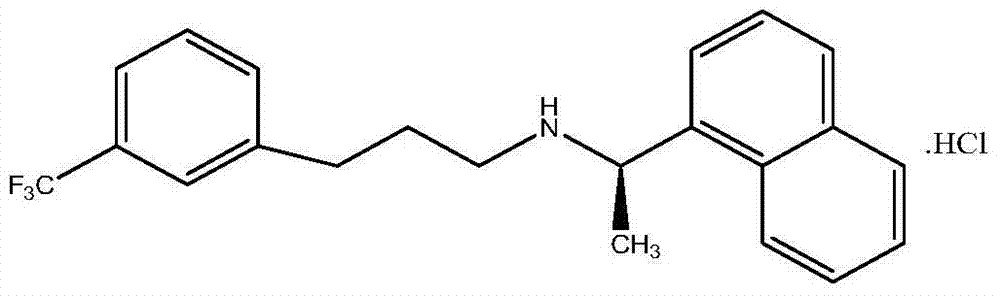
Synthetic method of cinacalcet hydrochloride and intermediate compound of cinacalcet hydrochloride
A technology of cinacalcet hydrochloride and a synthesis method, which is applied in the field of synthesis of cinacalcet hydrochloride and its intermediate compounds, can solve the problems affecting the quality of the cinacalcet hydrochloride product, the low free base yield and purity, Product market competitiveness and other issues, to achieve the effect of reducing product cost, improving yield and purity, and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
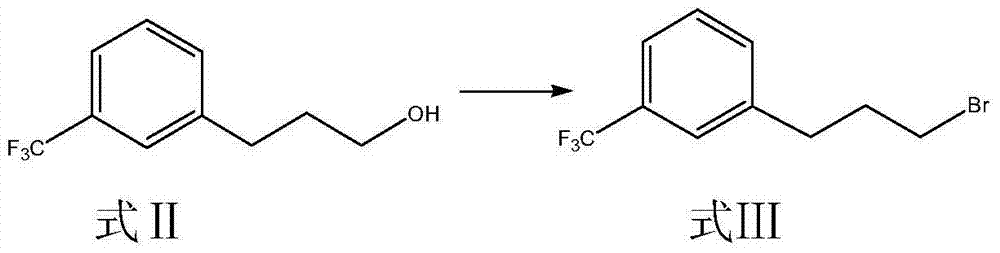
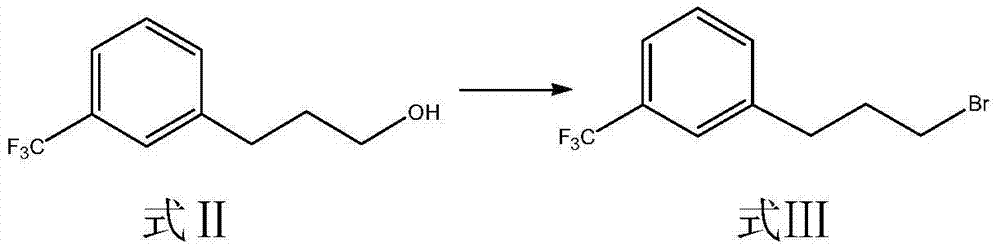
[0045]Synthesis of embodiment 1 m-trifluoromethyl phenylpropyl bromide (formula III compound)
[0046] Add 208.9g (1.023mol) of 3-[3-(tri-fluoromethyl)-phenyl]propanol (Formula II), 338g (2.005mol) of 48% hydrobromic acid and tetrabutylsulfuric acid into a 1000ml three-necked flask After 20g (0.0589mol) of ammonium hydrogen, stir and heat up until the temperature of the solution in the reaction bottle is stable at 82±2°C, weigh 402g (1.998mol) of 98% concentrated sulfuric acid in a 500ml constant pressure dropping funnel, and pour it slowly into the reaction bottle Slowly add concentrated sulfuric acid, control the dropping speed, keep the temperature in the reaction bottle at 82±2°C, drop it in about 3 hours, keep the temperature in the bottle, react for 22 hours, TLC (developing agent: petroleum ether / ethyl acetate =10:1) Monitor the completion of the reaction, cool down to room temperature, pour the reaction solution into a 2L separatory funnel, and after standing still, se...
Embodiment 2
[0048] The synthetic method of embodiment 2 cinacalcet hydrochloride
[0049] Step 1: Prepare the intermediate compound of formula III according to the method of Example 1 to obtain 248.6g;
[0050] Step 2: Synthetic formula V compound
[0051] Add 187.3g (1.0939mol) R-(+)-1-naphthylethylamine (Formula IV), 252g (1.832mol) anhydrous potassium carbonate, 0.36g potassium iodide, and 2.4L acetonitrile in a 3L three-necked reaction flask, and react Heat the system to an internal temperature of 60-70°C, then add 248.5g of the refined intermediate (Formula III) within 1 hour, keep the temperature in the reaction bottle at 60-70°C, react for 22 hours, and detect by TLC (developing agent: petroleum Ether / ethyl acetate=10:1) reaction solution, the intermediate (Formula III) basically reacted completely, the reaction solution was filtered and concentrated to recover acetonitrile to obtain cinacalcet free base oil; dissolve the oil in ethyl acetate , add 1mol / L hydrochloric acid soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com