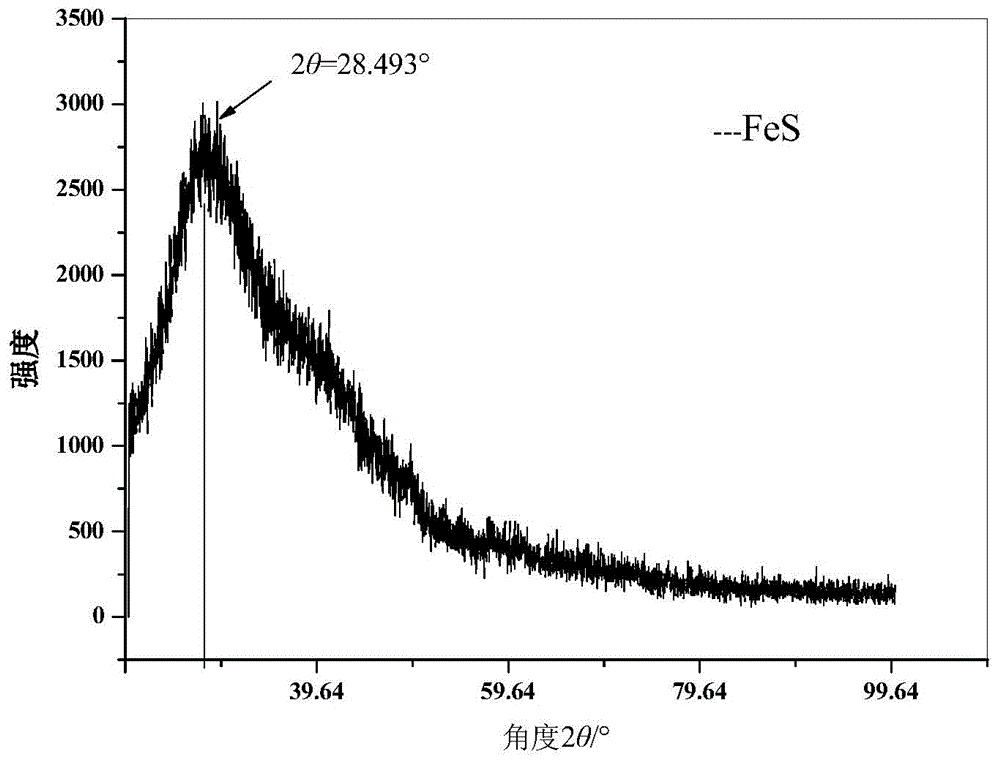
Preparation and use method of FeS for repairing hexavalent chromium pollution underground water
A groundwater, hexavalent chromium technology, applied in water/sewage treatment, chemical instruments and methods, reduced water/sewage treatment, etc., can solve the problems of many impurities, imperfect properties, unfavorable engineering use, etc., to achieve excellent properties, FeS Excellent properties and strict quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
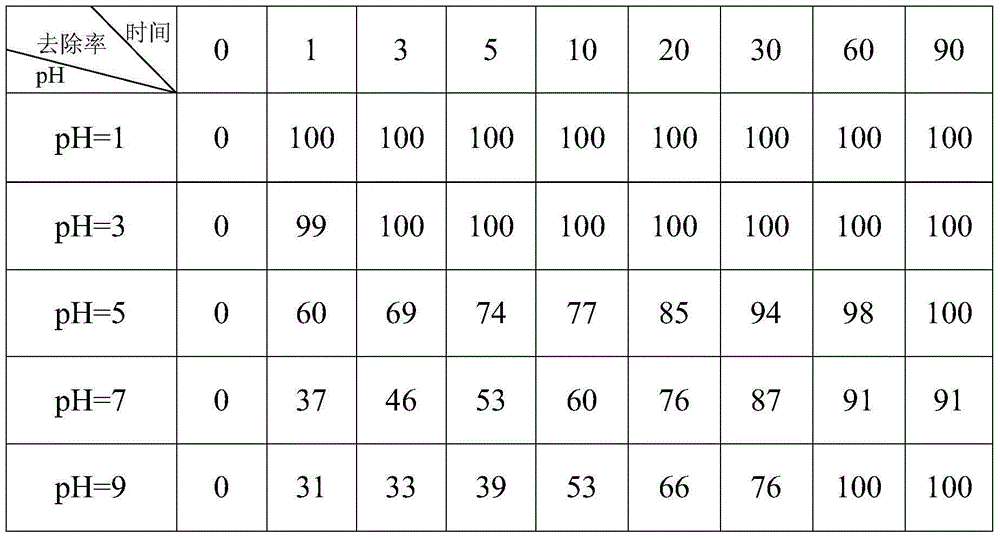
[0040] The following experiments were designed to study the application of different pH to FeS removal of hexavalent chromium in simulated groundwater, and the reaction was carried out in a numerically controlled constant temperature shaking incubator. First draw 100mL of an aqueous solution containing 10mg / L hexavalent chromium into a 250mL glass bottle, then add 2mL of FeS suspension containing 43.96mg / L into a 250mL glass bottle, the pH value of the hexavalent chromium solution ranges from 1 to 9 . Cover the rubber plug, put it into a constant temperature shaking incubator, control the reaction temperature at 20°C, and the rotation speed at 150r / min, draw 2mL of the solution with a syringe at regular intervals, and measure the residual hexavalent in the aqueous solution at different intervals (min). Chromium concentration, calculate the removal rate of hexavalent chromium (see Table 2).
[0041] The removal rate (%) of FeS to hexavalent chromium under the different pH cond...
Embodiment 2
[0044] The following experiments were designed to study the application of different temperatures on FeS removal of hexavalent chromium in simulated groundwater, and the reaction was carried out in a numerically controlled constant temperature shaking incubator. First draw 100mL of an aqueous solution containing 10mg / L hexavalent chromium into a 250mL glass bottle, then add 2mL of FeS suspension containing 43.96mg / L into a 250mL glass bottle, the pH of the hexavalent chromium solution is about 6.80. Cover the rubber plug, put it into a constant temperature shaking incubator, control the reaction temperature at 10°C (15°C, 20°C and 25°C), and the rotation speed at 150r / min, draw 2mL of the solution with a syringe at regular intervals, and measure different time (min) Interval concentration of hexavalent chromium in the aqueous solution to calculate the removal rate of hexavalent chromium (see Table 3).
[0045] Table 3 Removal rate (%) of FeS to hexavalent chromium under differ...
Embodiment 3
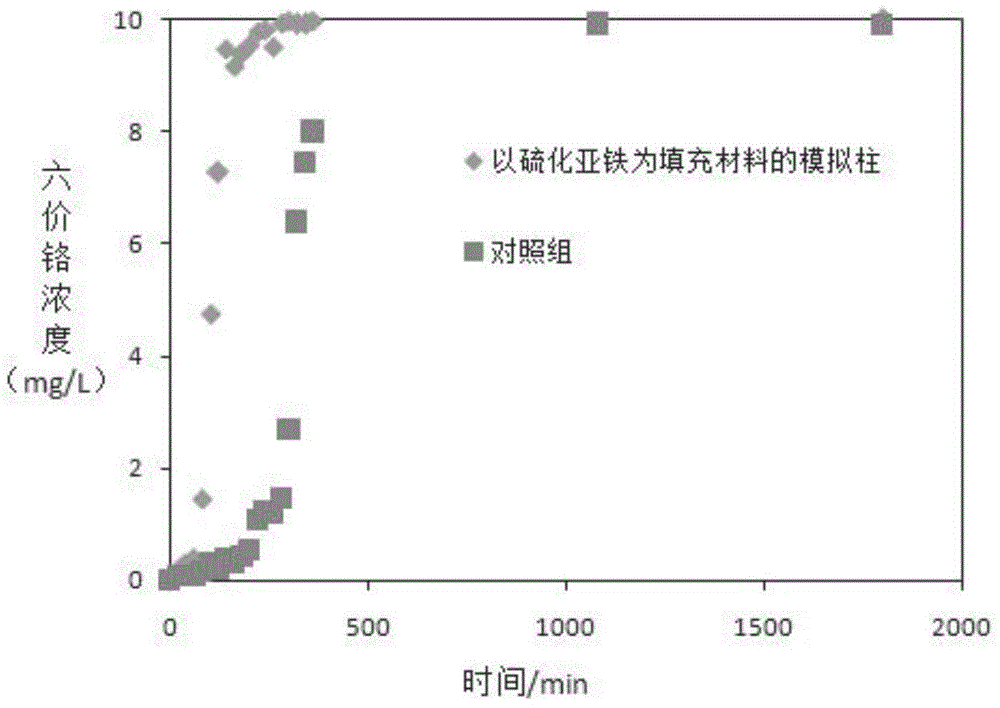
[0048] The following column experiment was designed to preliminarily study the application of FeS suspension to remove hexavalent chromium in simulated groundwater under dynamic conditions. Choose a plexiglass column with a height of 30 cm and an inner diameter of 8 cm. The filling material used in the experiment is medium sand (with a particle size between 0.25 and 0.50 mm). Before the experiment started, the glass column was saturated with water from top to bottom for about two hours, and then the glass column was placed horizontally. Then 20 mL of freshly prepared FeS suspension (25 g / L) was injected into the middle and bottom of the experimental column, and the same volume of deionized water was injected into the control experiment. Inject 10 mg / L hexavalent chromium solution from the left end through a peristaltic pump at a flow rate of 1 ml / min. Measure the concentration of hexavalent chromium at the outlet when running at different times, the results are as follows f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com