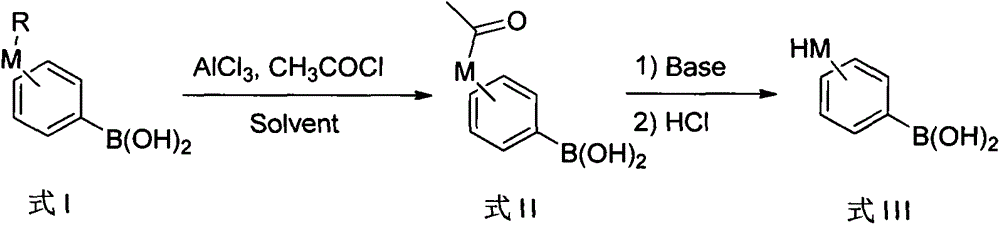
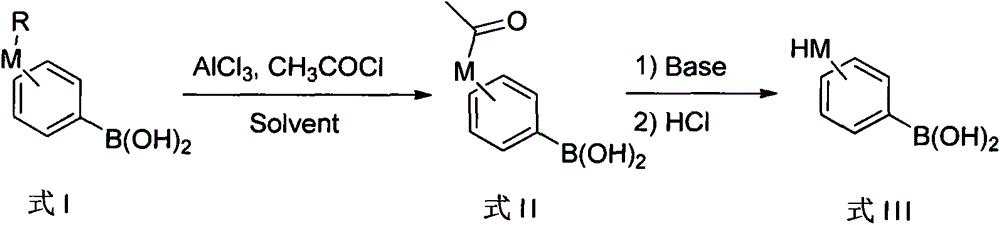
A method of preparing phenylboronic acid ortho- meta- and para-substituted with hydroxy and mercapto
A technology of mercaptophenylboronic acid and hydroxyl group, which is applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as inability to obtain pure products, and achieve the effects of high yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Synthesis of o-hydroxyphenylboronic acid:
[0020] Under the protection of nitrogen, 1.5 kg of 2-methoxyphenylboronic acid, 18 liters of anisole and 1.56 kg of acetyl chloride were added into a 30L glass reactor. After the addition, the reaction solution was cooled to -10-0°C and stirred for 1 hour. Begin to add 133 grams of anhydrous aluminum trichloride in batches. After the addition is completed and naturally rise to room temperature, keep warm and stir the reaction until TLC shows that the raw materials are completely reacted. This process takes about 8 to 10 hours. During this process, the solids of the reaction mixture were gradually dissolved, followed by the gradual precipitation of solids.
[0021] Add 5M potassium hydroxide aqueous solution to quench and adjust the pH to 11-12. At this point the organic layer was separated and discarded. Add 6M hydrochloric acid aqueous solution to the water layer to adjust the pH to 2-3, extract twice with 12 liters of ethy...
Embodiment 2
[0023] Synthesis of p-hydroxyphenylboronic acid:
[0024] Under the protection of nitrogen, add 1.5 kg of 4-methoxyphenylboronic acid, 20 liters of toluene and 1.17 kg of acetyl chloride into a 30L glass reactor. After the addition, cool the reaction solution to -10-0°C and stir for 1 hour. Begin to add 267 grams of anhydrous aluminum trichloride in batches, rise to 80 ° C after the addition is complete, keep warm and stir the reaction until TLC shows that the raw materials have reacted completely, and this process takes about 3 to 5 hours. During this process, the solids of the reaction mixture were gradually dissolved, and then solids were gradually precipitated out.
[0025] Add 2M sodium hydroxide aqueous solution to quench and adjust the pH to 11-12. At this point the organic layer was separated and discarded. Add 6M hydrochloric acid aqueous solution to the water layer to adjust the pH to 2, extract twice with 15 liters of ethyl acetate, combine the organic layers, was...
Embodiment 3
[0027] Synthesis of p-mercaptophenylboronic acid:
[0028] Under nitrogen protection, add 4.2 kg of 4-tert-butylmercaptophenylboronic acid, 25 liters of diphenyl ether and 2.3 kg of acetyl chloride into a 50L glass reactor. After the addition, cool the reaction solution to -10-0°C and stir for 1 hour. Begin to add 267 grams of anhydrous aluminum trichloride in batches, add complete. Raise the reaction solution to 80°C, keep warm and stir the reaction until TLC shows that the reaction of the raw materials is complete, and this process takes about 2 to 3 hours. During this process, the solids of the reaction mixture were gradually dissolved, and then solids were gradually precipitated out.
[0029] Add 2M sodium hydroxide aqueous solution to quench and adjust the pH to 11-12. At this point the organic layer was separated and discarded. Add 6M hydrochloric acid aqueous solution to the water layer to adjust the pH to 2-3, extract twice with 15 liters of ethyl acetate, combine t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com