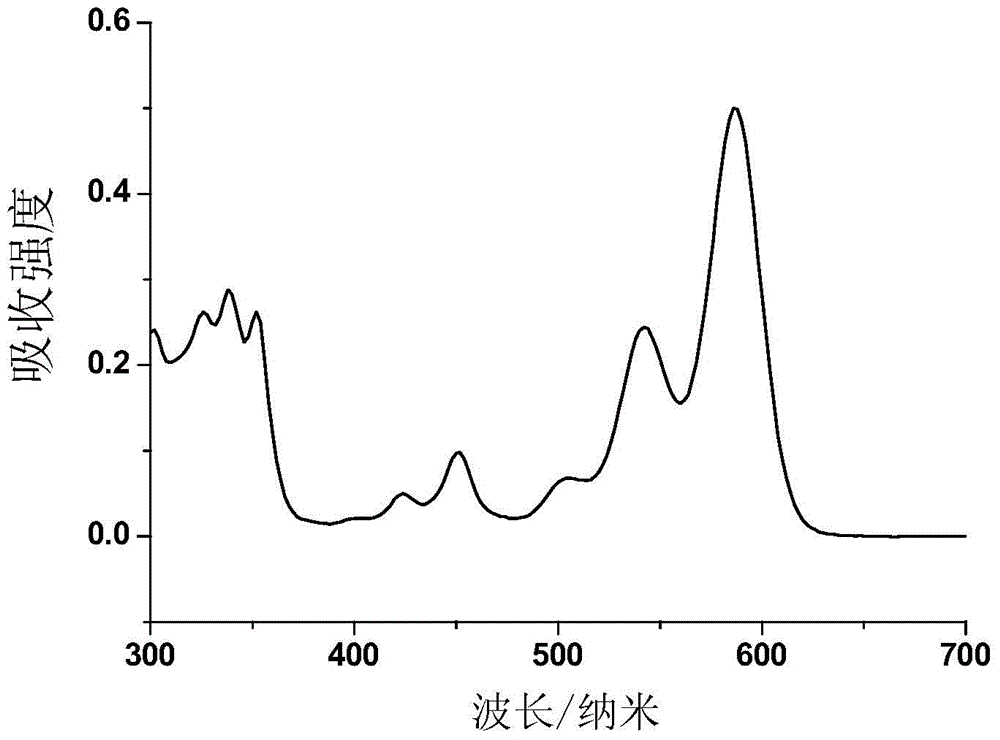
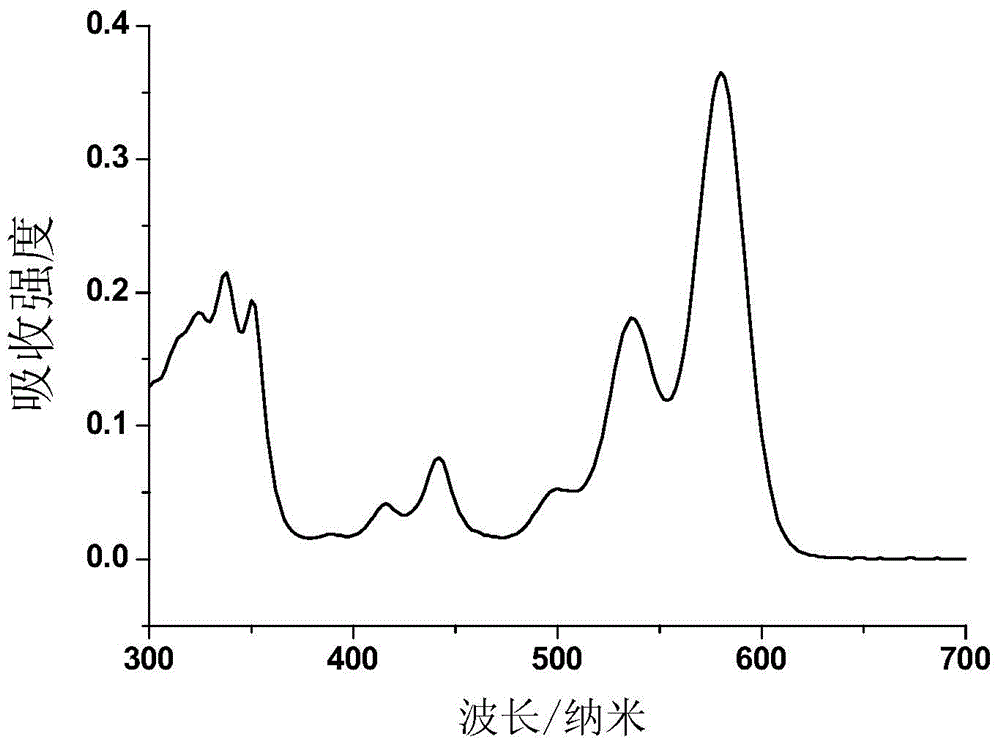
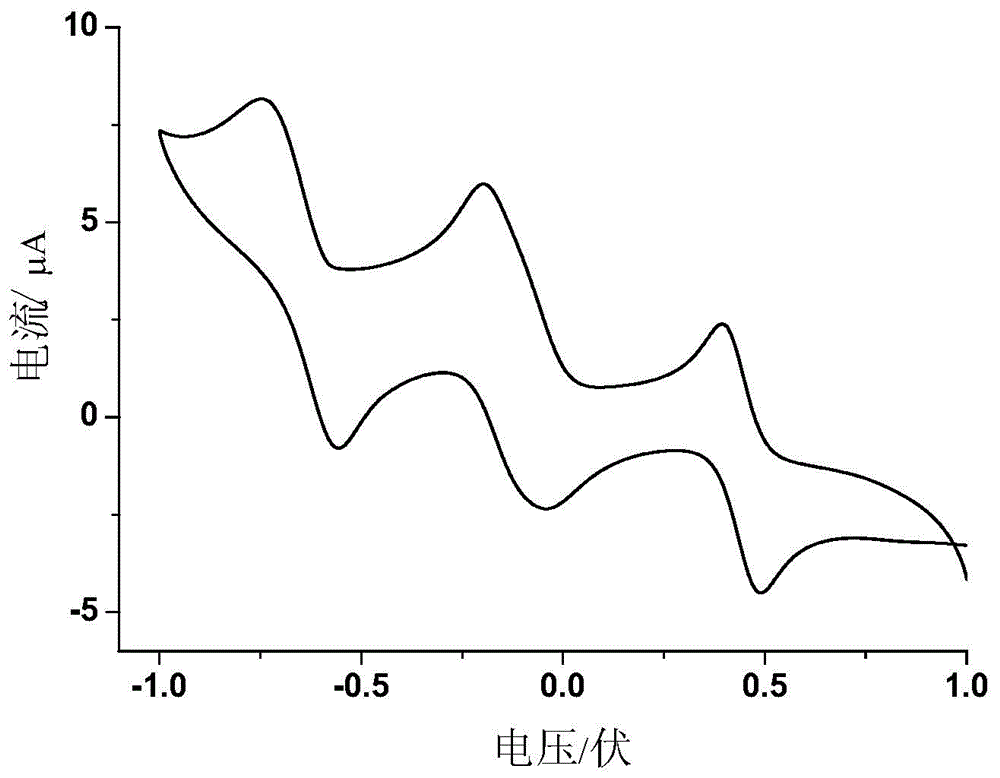
Selenium-containing pi extension naphthalene tetracarboxylic diimide compound, and preparation method and application thereof
A technology of naphthalene tetracarboxylic acid diimide and compound, which is applied in the field of naphthalene tetracarboxylic acid diimide derivatives, and can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] N,N′-bis(2-butyl-octyl)-[2,3-d:6,7-d′]-bis[1,3-diseleno-2-yrylene-propanedicyanide Synthesis of ]-naphthalene-1,4,5,8-tetracarboxylic diimide (compound 1)
[0093] Concrete synthetic steps are:
[0094]Under the protection of nitrogen, the compound N,N′-bis(2-butyl-octyl)-2,3,6,7-tetrabromonaphthalene-1,4,5,8-tetracarboxylic diimide Amine (918mg, 1.00mmol), potassium 1,1-dicyanoethylene-2,2-diselenate (700mg, 2.50mmol) were added into 160mL THF, heated to 50°C, and stirred for 2.5 hours. Cool to room temperature, spin off the solvent under reduced pressure, use dichloromethane / petroleum ether (2 / 1) as eluent, separate and purify the crude product with silica gel chromatography, and obtain 448 mg of blue-purple solid product (compound 1), Yield 42%.
[0095] Mass Spectrum: [MS(TOF)]m / z:1068.1(M+H) + .
[0096] Elemental analysis: Molecular formula: C 46 h 50 N 6 o 4 Se 4 ; Theoretical: C, 51.79; H, 4.72; N, 7.88; Found: C, 51.76; H, 4.77; N, 7.76.
[0097] Pro...
Embodiment 2
[0100] N,N′-bis(2-octyl-dodecyl)-[2,3-d:6,7-d′]-bis[1,3-diseleno-2-yaryne-propane Synthesis of dicyano]-naphthalene-1,4,5,8-tetracarboxylic diimide (compound 2)
[0101] Concrete synthetic steps are:
[0102] Replace N with N,N′-bis(2-octyl-dodecyl)-2,3,6,7-tetrabromonaphthalene-1,4,5,8-tetracarboxylic diimide, N'-bis(2-butyl-octyl)-2,3,6,7-tetrabromonaphthalene-1,4,5,8-tetracarboxylic diimide, the synthesis method is the same as in Example 1 step, a blue-purple solid (compound 2) was prepared with a yield of 28%.
[0103] Mass Spectrum: [MS(TOF)]m / z:1292.5(M+H) + .
[0104] Elemental analysis: Molecular formula: C 62 h 82 N 6 o 4 Se 4 ; Theoretical: C, 57.67; H, 6.40; N, 6.51; Found: C, 57.66; H, 6.39; N, 6.31.
[0105] H NMR spectrum: 1 H-NMR (300MHz, CDCl 3 )δ(ppm):0.85–0.87(m,6H,–CH 3 ),1.25(m,32H,–CH 2 –),2.01(m,1H,CH),4.23(d,2H,J=7.50Hz,–CH 2 –N).
[0106] After testing, compound 2 is soluble in common organic solvents, such as dichloromethane, chloroform...
Embodiment 3
[0108] N,N′-bis(3-hexyl-undecyl)-[2,3-d:6,7-d′]-bis[1,3-diseleno-2-yaryne-propanedi Synthesis of cyano]-naphthalene-1,4,5,8-tetracarboxylic diimide (compound 3)
[0109] Concrete synthetic steps are:
[0110] Use N,N′-bis(3-hexyl-undecyl)-2,3,6,7-tetrabromonaphthalene-1,4,5,8-tetracarboxylic diimide instead of N,N ′-bis(2-butyl-octyl)-2,3,6,7-tetrabromonaphthalene-1,4,5,8-tetracarboxylic diimide, the synthesis method is the same as that in Example 1 , a blue-purple solid (compound 3) was prepared with a yield of 28%.
[0111] Mass Spectrum: [MS(TOF)]m / z:1206.3(M + ).
[0112] Elemental analysis: Molecular formula: C 56 h 70 N 6 o 4 Se 4 ; Theoretical: C, 55.72; H, 5.85; N, 6.96; Found: C, 55.99; H, 5.80; N, 7.05.
[0113] H NMR spectrum: 1 H-NMR (300MHz, CDCl 3 )δ(ppm):0.85–0.94(m,6H,–CH 3 ),1.23–1.33(m,24H,–CH 2 –),1.52(br,1H,CH),1.74(br,2H,–CH 2 –),4.02–4.06(m,2H,–CH 2 –N).
[0114] After testing, compound 3 is soluble in common organic solvents, such as dic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com