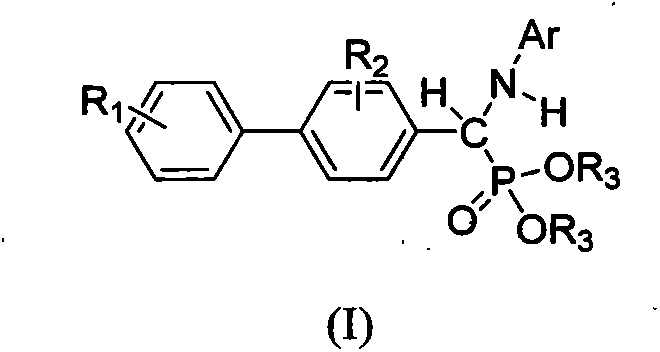
Biphenyl alpha-aminophosphonate compound as well as preparation method and application thereof
A technology of amino phosphonate and methyl phosphonate, applied in the field of drug synthesis, can solve problems such as unsatisfactory control effects, and achieve the effects of mild reaction conditions, easy availability of raw materials, and simple operation of the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
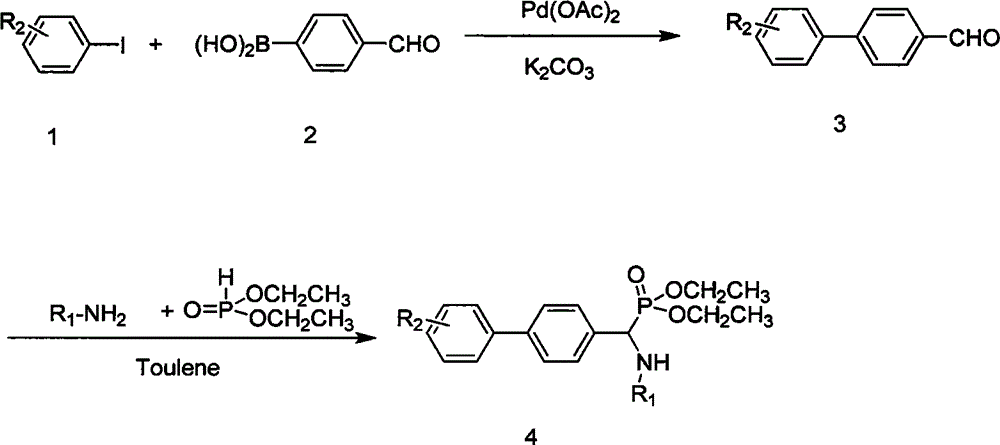
[0045] Compound O, the synthesis of O'-diethyl-1-(phenylamino)-1-(4-biphenyl)methylphosphonate (4a):
[0046] (1) Synthesis of 4-biphenyl formaldehyde:
[0047] Add potassium carbonate (0.212g, 3mmol), palladium acetate (2mg, 0.03mmol), polyethanol 2000 (3.5g) and water (3mL) into a 25mL round bottom flask, heat the system to 50°C and stir well, then add p-Aldehydophenylboronic acid (0.225g, 1.5mmol) and iodobenzene (0.204g, 1mmol) were placed in a reaction flask, under the protection of nitrogen, the reaction was carried out for 12h, cooled to room temperature after the reaction, the reaction product was filtered with toluene (3 × 10mL) to extract the filtrate, the extracted organic phase was washed and dried, and the solvent was evaporated, and the white solid biphenyl formaldehyde was obtained by recrystallization from petroleum ether, with a yield of 93% and a melting point of 54-55°C;
[0048] (2) Compound O, the synthesis of O'-diethyl-1-(phenylamino)-1-(4-biphenyl)meth...
Embodiment 2
[0051] Compound O, O'-diethyl-1-(phenylamino)-1-(4'-methoxy-4-biphenyl)methylphosphonate (4b)
[0052] (1) Synthesis of 4'-methoxyl 4-biphenyl formaldehyde:
[0053] The synthesis method and conditions are the same as those in Example 1(1). The difference is that 4-methoxyiodobenzene (234mg, 1.0mmol) was added, the reaction time was 12h, the yield was 96%, and the melting point was 102-103°C.
[0054] (2) Compound O, the synthesis of O'-diethyl-1-(phenylamino)-1-(4'-methoxy-4-biphenyl)methylphosphonate:
[0055] The synthesis method and conditions are the same as those in Example 1(2). The difference is that 4'-methoxy 4-biphenylcarbaldehyde (106mg, 0.5mmol) was added, the reaction time was 6h, the yield was 83%, and the melting point was 101-103°C.
Embodiment 3
[0057] Compound O, O'-diethyl-1-(4-methylanilino)-1-(4'-methoxy-4-biphenyl)methylphosphonate (4c)
[0058] (1) Synthesis of 4'-methoxyl 4-biphenyl formaldehyde:
[0059] The synthesis method and conditions are the same as those in Example 2(1).
[0060] (2) Compound O, the synthesis of O'-diethyl-1-(4-methylanilino)-1-(4'-methoxy-4-biphenyl)methylphosphonate:
[0061] The synthesis method and conditions are the same as those in Example 2(2). The difference is that 4-methylaniline (54mg, 0.5mmol) was added, the reaction time was 6h, the yield was 86%, and the melting point was 151-153°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com