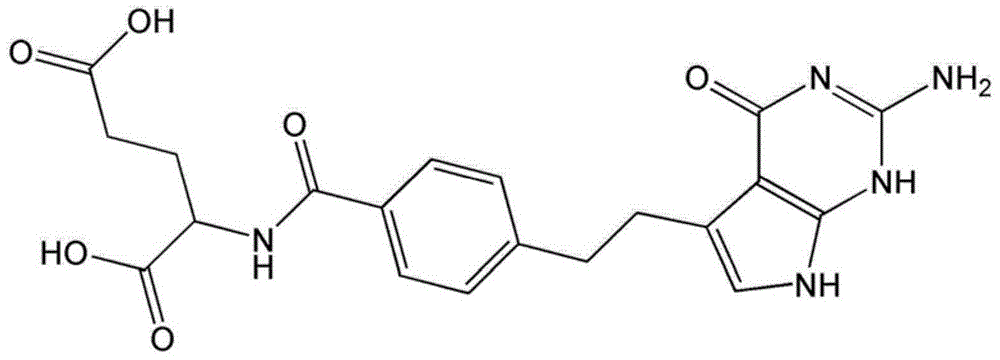
A stabilized pemetrexed formulation
A pemetrexed preparation technology, applied in the field of stable pemetrexed preparations, can solve problems such as insufficient stability, achieve the effects of preventing microbial contamination, and improving convenience and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 14
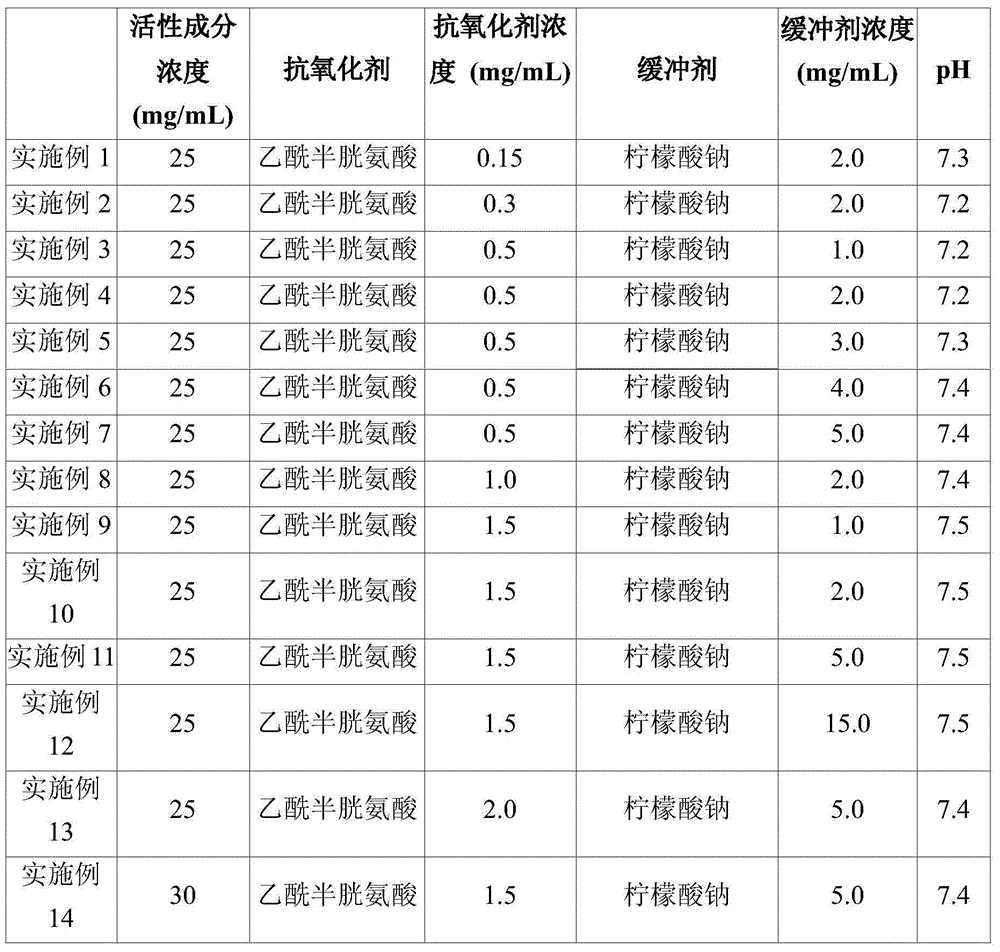
[0049] Examples 1 to 14: Containing different amounts of acetylcysteine as Preparation of Injectable Solution Containing Pemetrexed with Antioxidant and Sodium Citrate as Buffer
[0050] 2.5 g of D-mannitol was dissolved in 100 ml of WFI (Water for Injection), and then acetylcysteine and sodium citrate were continuously added thereto at the concentrations shown in Table 1 below and completely dissolved therein. 2.5 g of pemetrexed was slowly added to the solution (in Example 14 3.0 g of pemetrexed was added slowly) and the solutions were stirred until they became clear. Then, the solution was adjusted to the pH shown in Table 1 with aqueous hydrochloric acid or sodium hydroxide. The solution was sterile filtered through a sterile 0.22-μm membrane filter in a clean room. The obtained solution was poured into previously washed / sterilized sealable containers purged with nitrogen.
[0051] The composition and pH of the obtained injectable solution containing pemetrexed a...
Embodiment 15 and 16
[0054] Examples 15 and 16: Preparation of injectable solutions containing pemetrexed with different pH
[0055] 2.5 g of D-mannitol was dissolved in 100 ml of WFI (Water for Injection), and then acetylcysteine and sodium citrate were continuously added thereto at the concentrations shown in Table 2 below and completely dissolved therein. 2.5 g of pemetrexed was slowly added to the solution and the solutions were stirred until they became clear. Then, the solution was adjusted to the pH shown in Table 2 with aqueous hydrochloric acid or sodium hydroxide. The solution was sterile filtered through a sterile 0.22-μm membrane filter in a clean room. The obtained solution was poured into previously washed / sterilized sealable containers purged with nitrogen.
[0056] The composition and pH of the obtained injectable solution containing pemetrexed are shown in Table 2 below.
[0057] Table 2
[0058]
experiment Embodiment 1
[0075] Experimental Example 1: Stress stability test (60°C / 80%, 4-week evaluation)
[0076] The stress stability test was performed as described above and the test results are shown in Tables 7 to 11 below.
[0077] and also, figure 1 The injectable liquid formulation containing pemetrexed according to the present invention (Example 11) and the state of the art containing pemetrexed were shown during the 4-week stability test period under stress test conditions (60°C and 80%) Comparison of appearance between the injectable liquid formulations (Comparative Examples 1, 12 and 14) of . For comparison, the appearance of WFI (Water for Injection) is also shown in figure 1 middle.
[0078] Table 7
[0079]
[0080]
[0081]
[0082] As can be seen from the results in Table 7, formulations containing 1.5 mg / mL acetylcysteine as an antioxidant and 1-15 mg / mL sodium citrate as a buffer showed 0.2% or less The individual impurity content and the total impurity content ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com