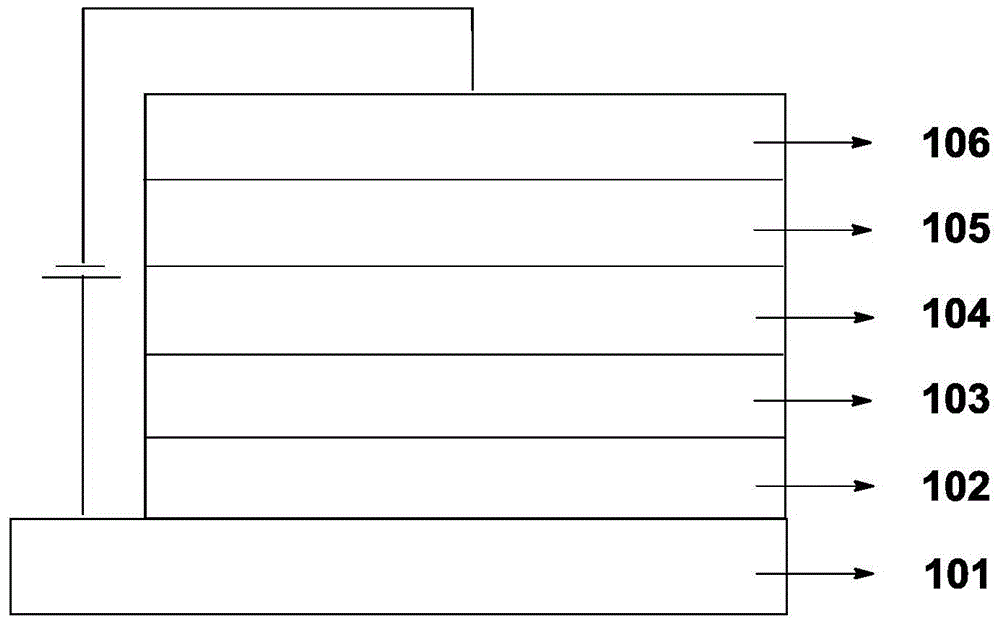
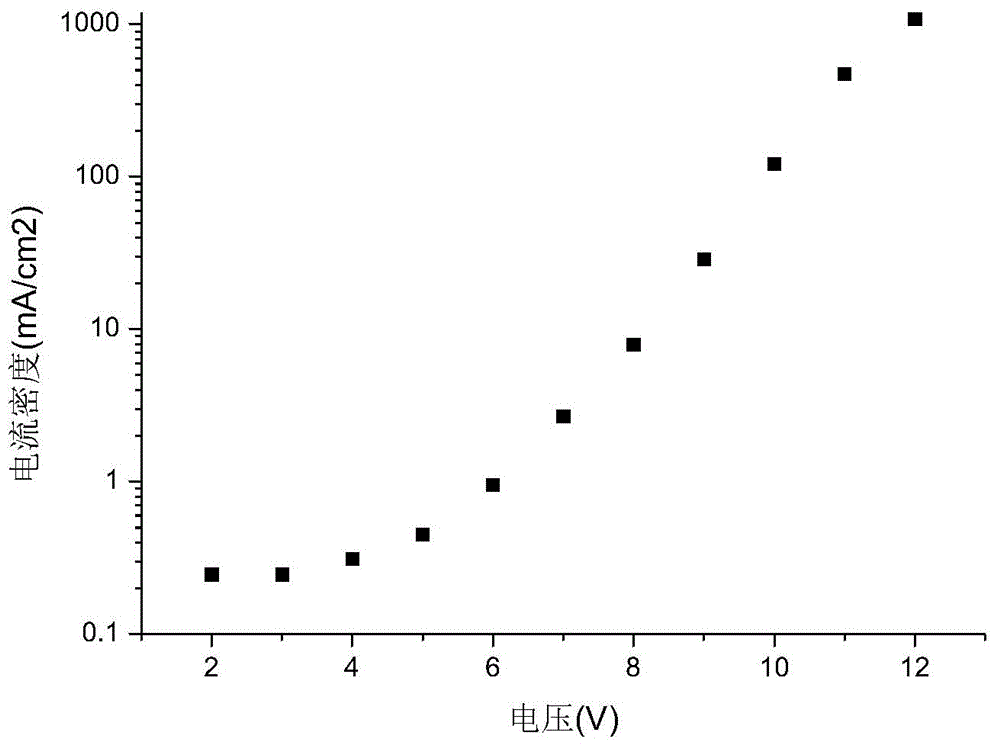
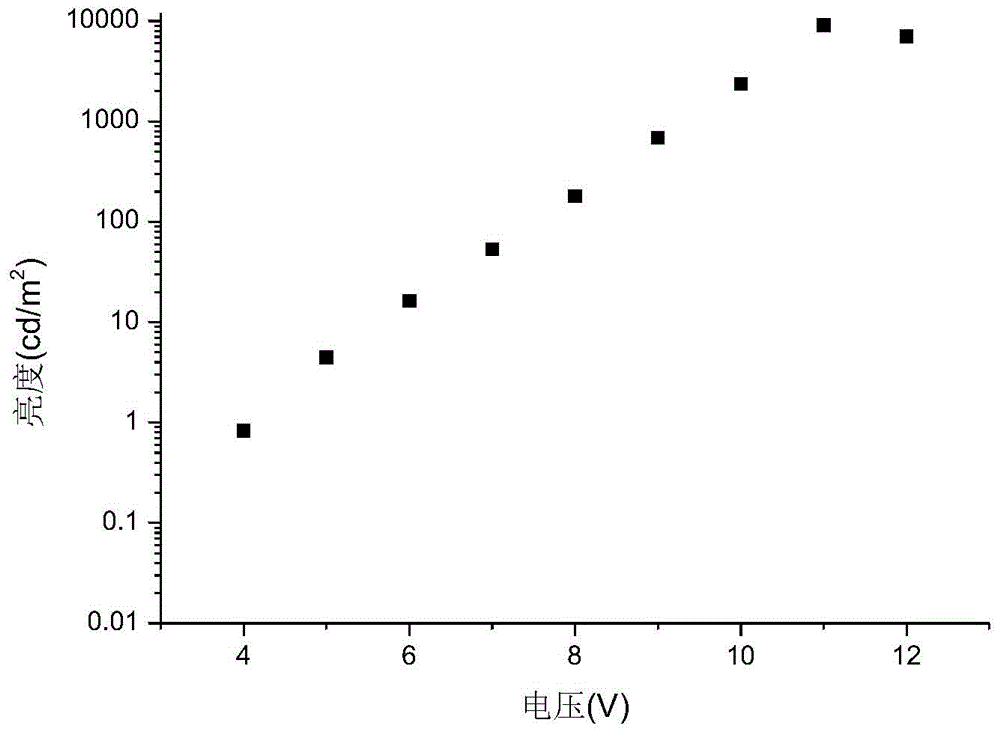
Organic electroluminescence material and application thereof
An electroluminescent material and electroluminescent technology, applied in the fields of luminescent materials, organic chemistry, circuits, etc., can solve the problem of low activity, and achieve the effect of good device performance, good efficiency and good film stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1 intermediate compound 4
[0047]
[0048] Preparation of Compound 1: In a 5L three-necked flask, add naphthyl-1,5-dicarbaldehyde (92g, 0.5mol), methoxymethyltriphenylphosphine chloride (410g, 1.2mol), toluene (1800g), N 2Protection, lower the temperature to the temperature in the reaction bottle <-5°C, dissolve potassium tert-butoxide (135g, 1.2mol) in 520g THF, slowly drop into the three-necked flask, control the temperature in the bottle to <5°C, and complete the dropwise addition in 0.5h , -5 ℃ ~ 5 ℃ insulation reaction for 3h. Add 600g of deionized water, stir for 10min, separate liquid, wash the organic phase with 600mL X 2 water, remove toluene under reduced pressure, add 1400g of petroleum ether, stir for 0.5h, filter with suction, rinse the filter cake with 400g of petroleum ether, collect the filtrate, remove Petroleum ether was used to obtain compound 1, with a total weight of 97.5 g, a yield of 81.3%, MS (m / s): 240.1, and ...
Embodiment 2
[0052] The preparation of embodiment 2 intermediate compound 6
[0053]
[0054] Using cyclopentenyl trimethylsilyl ether instead of cyclohexenyl trimethylsilyl ether, compound 6 was prepared according to the method described in Example 1, and the yields of the two-step reactions were 21.9% and 76.2%, respectively, to obtain compound 6 fine products 21.5g, high-resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 24 h 18 Br 2 , the theoretical value is 465.9755, and the test value is 465.9758.
Embodiment 3
[0055] The preparation of embodiment 3 target compound C01
[0056]
[0057] In a 250mL three-necked flask, add compound 4 (2.47g, 5mmol) prepared in Example 1, 2-naphthaleneboronic acid (2.1g, 12mmol), K 2 CO 3 (6.0g, 43mmol), toluene (80mL), deionized water (24mL), N 2 protection, adding Pd(PPh 3 ) 4 (320mg), warming up to reflux, reacting for 36 hours, stopping the reaction, cooling down, there is solid precipitation, suction filtration, 150mL deionized water rinses the filter cake, 50mL deionized ethanol rinses the filter cake, collects the filter cake, adds 300mL toluene, Heat up to reflux to obtain a clear liquid, quickly pass through a 10cm thick silica gel layer while it is hot, collect the filtrate, naturally cool down, and place it overnight, solids are precipitated, filtered by suction, and the target object C01 is obtained. The weight of the crude product is 2.08g. Purification by sublimation at a sublimation temperature of 360° C. to obtain 1.82 g of target...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com