Iron-zeolite chabazite catalyst for use in NOx reduction and method of making
A catalyst and zeolite-type technology, applied in the direction of catalyst activation/preparation, molecular sieve catalysts, chemical instruments and methods, etc., can solve problems such as insufficient thermal stability and damage to exothermic reactions of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
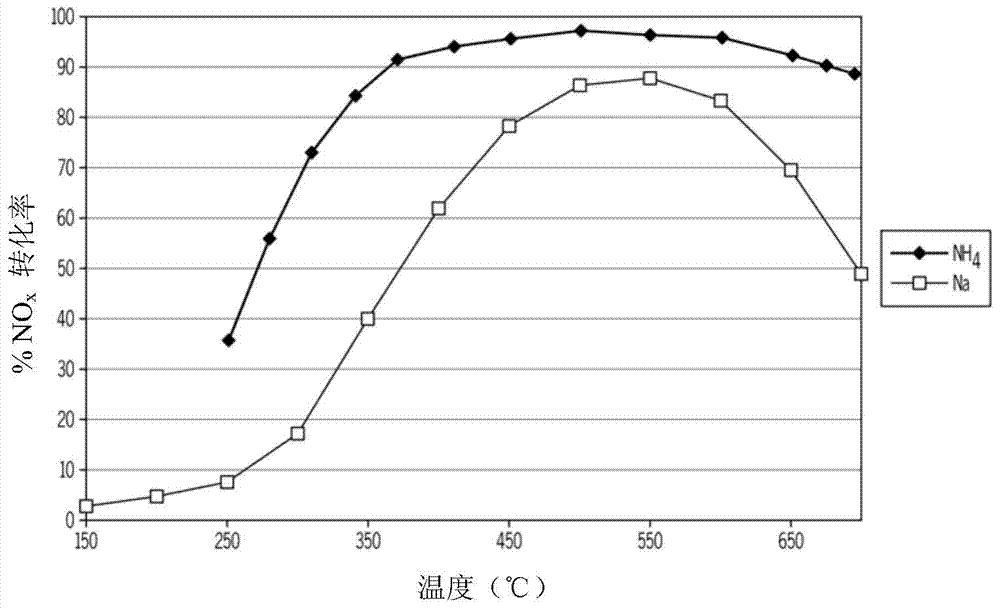
[0046] Chabazite samples were synthesized according to the procedure detailed above. The iron content was determined to be 1.2 wt% for both samples. One sample was ion exchange with ammonium ions to convert chabazite to the ammonium form. The sodium content in the non-exchanged sample was determined to be 6.3 wt%, whereas the sodium content after ammonium ion exchange was 390 ppm. Na and NH 4 The chabazite samples were then degreened at 750°C for 4 hours. Two samples were then used containing NO x The simulated vehicle exhaust device is tested. The samples were tested in a bench-top continuous reactor using simulated diesel engine exhaust from 13% O 2 , 5% CO 2 , 4.5%H 2 O, 350ppm NO, 350ppm NH 3 and balance N 2 composition. 3.0 grams of each sample was placed on an airflow of 9.65 SLPM (standard liters per minute). This corresponds to a space velocity of 30,000 / hour on the washed monolith. except N 2 and O 2 All other components were analyzed simultaneously by F...
Embodiment 2
[0049] Three different samples of the catalyst were prepared and tested. The first two samples were synthesized according to the procedure detailed above. For both samples, the iron content was determined to be 1.2 wt%. Both samples were then ion exchanged with ammonium ions to convert the chabazite to the ammonium form. The sodium content was greatly reduced after ammonium ion exchange. Two chabazite samples were then degreened at 750°C for 4 hours. A second chabazite sample was then subjected to accelerated aging at 800°C for 80 hours. A third sample comprised a conventional copper-based SCR catalyst obtained from BASF that had been degreened at 750°C for 4 hours. All samples were then used to contain NO x simulated vehicle exhaust for testing. After initial degreening at 750° C. for 4 hours, samples were tested in a benchtop continuous reactor. The simulated diesel exhaust is composed of 13% O 2 , 5% CO 2 , 4.5%H 2 O, 350ppmNO, 350ppmNH 3 , and the balance N 2 c...
Embodiment 3
[0052] The aged chabazite catalyst prepared according to the embodiment of the present invention was tested along with the degreened traditional copper-based SCR monolithic catalyst. The catalysts were tested individually and in combination with chabazite particulate catalyst positioned immediately upstream of the copper-based SCR monolith catalyst. Samples were tested in a benchtop continuous reactor after initial degreening at 750 °C for 4 hours. The simulated diesel exhaust is composed of 13% O 2 , 5% CO 2 , 4.5%H 2 O, 350ppm NO, 350ppm NH 3 , and the balance N 2 composition. 3.0 grams of chabazite pellets and 1.18in 3 The copper-based SCR monolith catalyst was placed on an airflow of 9.65 SLPM, corresponding to a space velocity of 30,000 / hour on each component. except N 2 and O 2All other gas species are analyzed simultaneously by FTIR. From Figure 5 It can be seen that the performance of the combined chabazite catalyst and copper-based catalyst is superior to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com