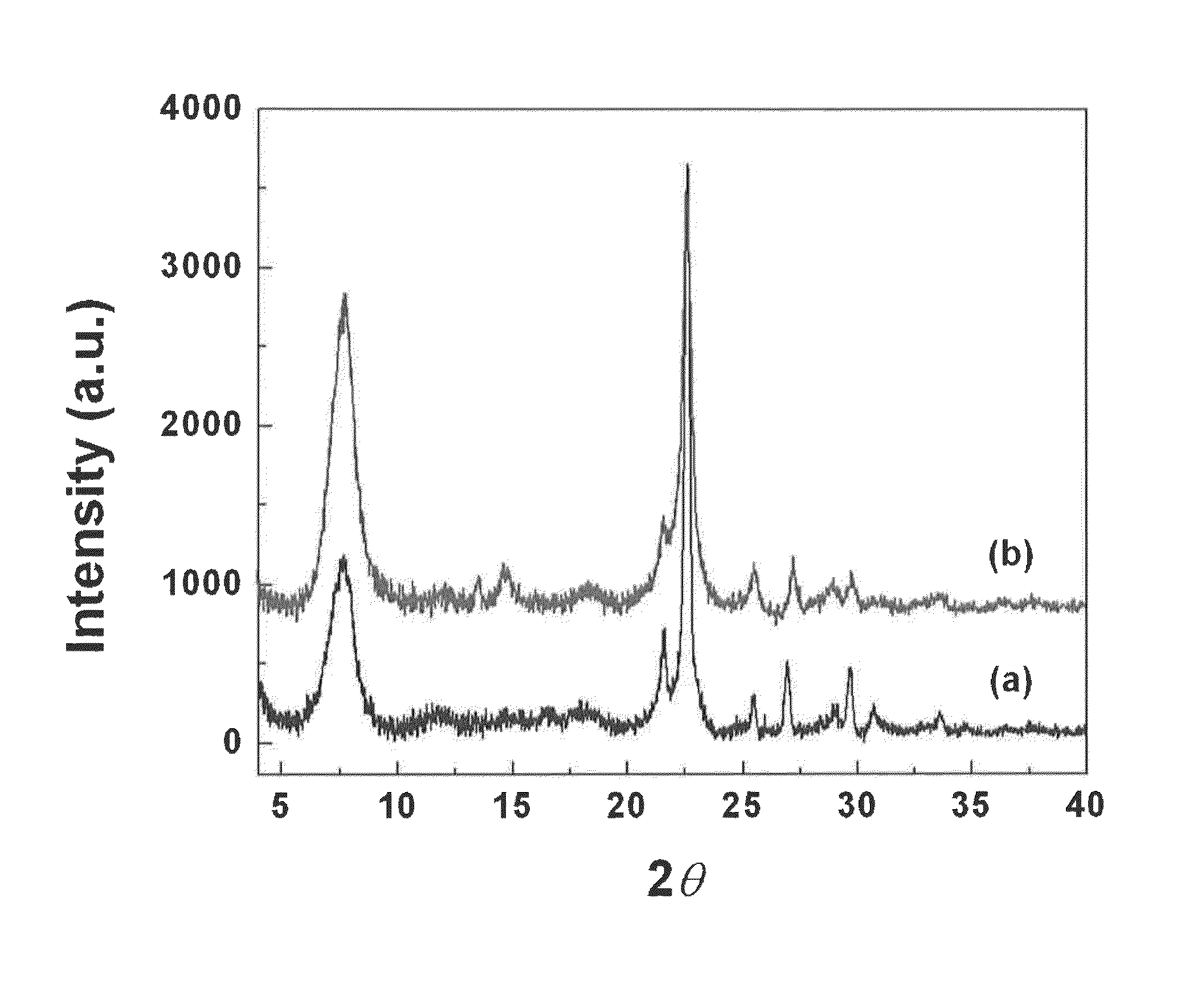
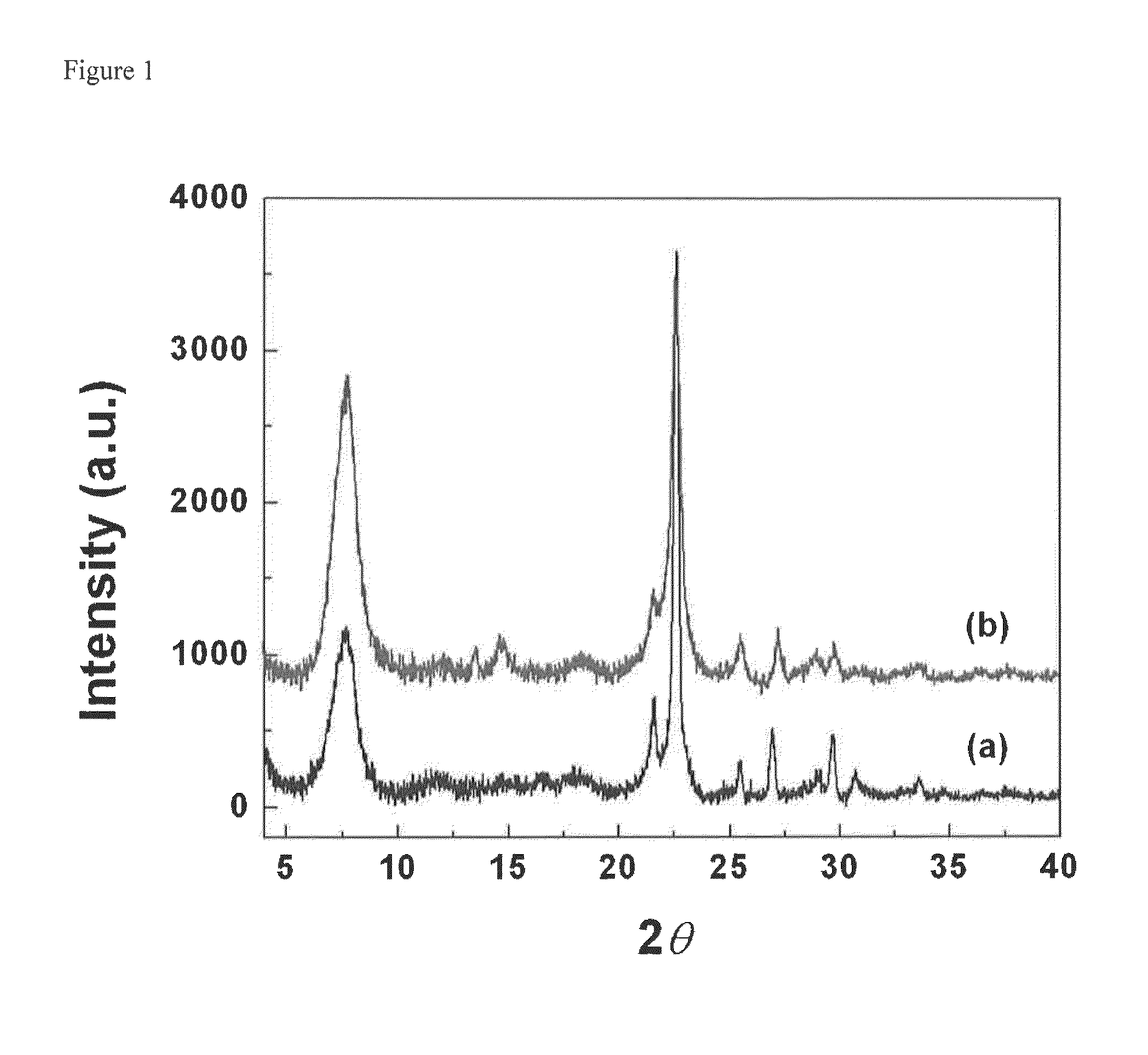
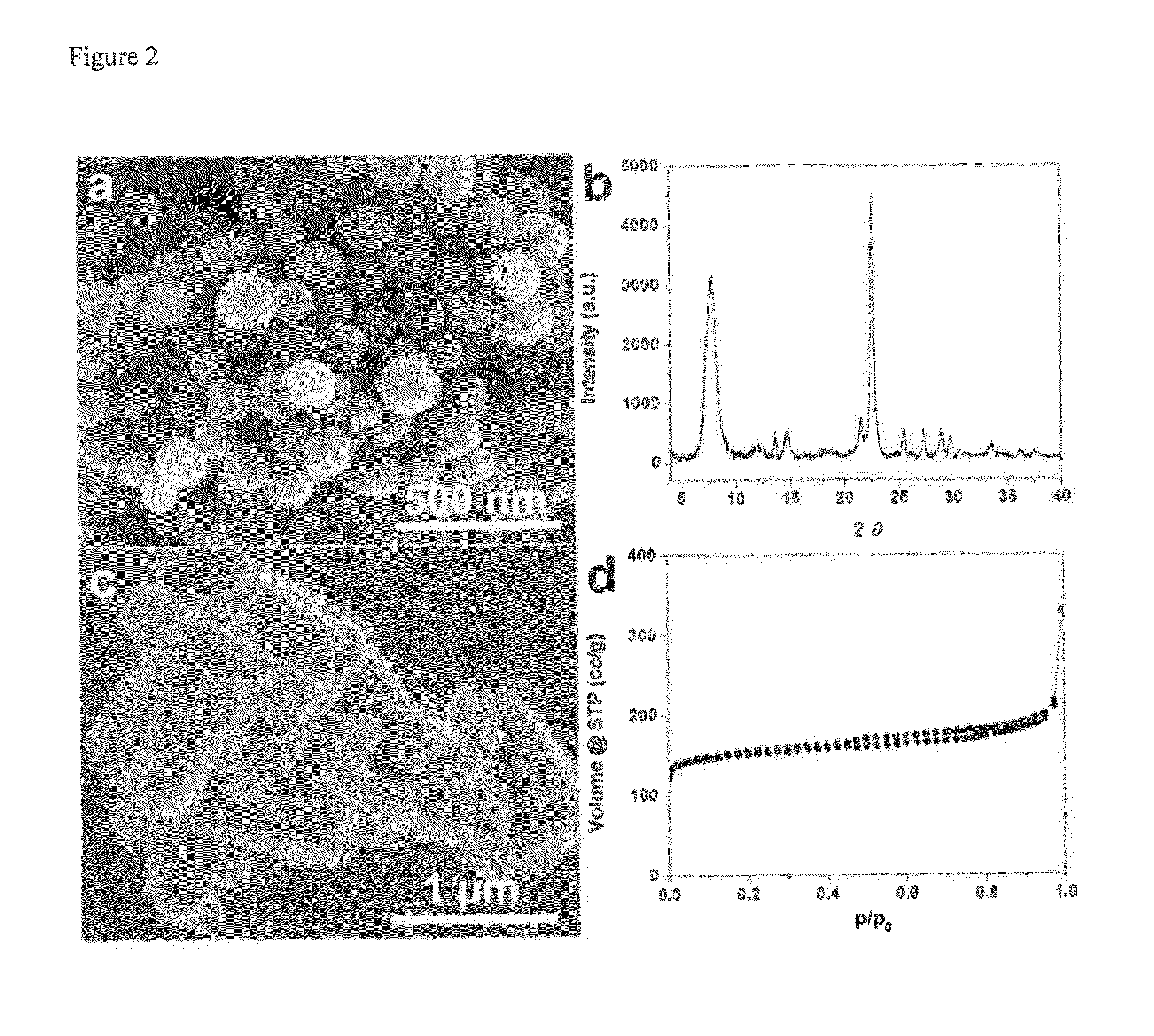
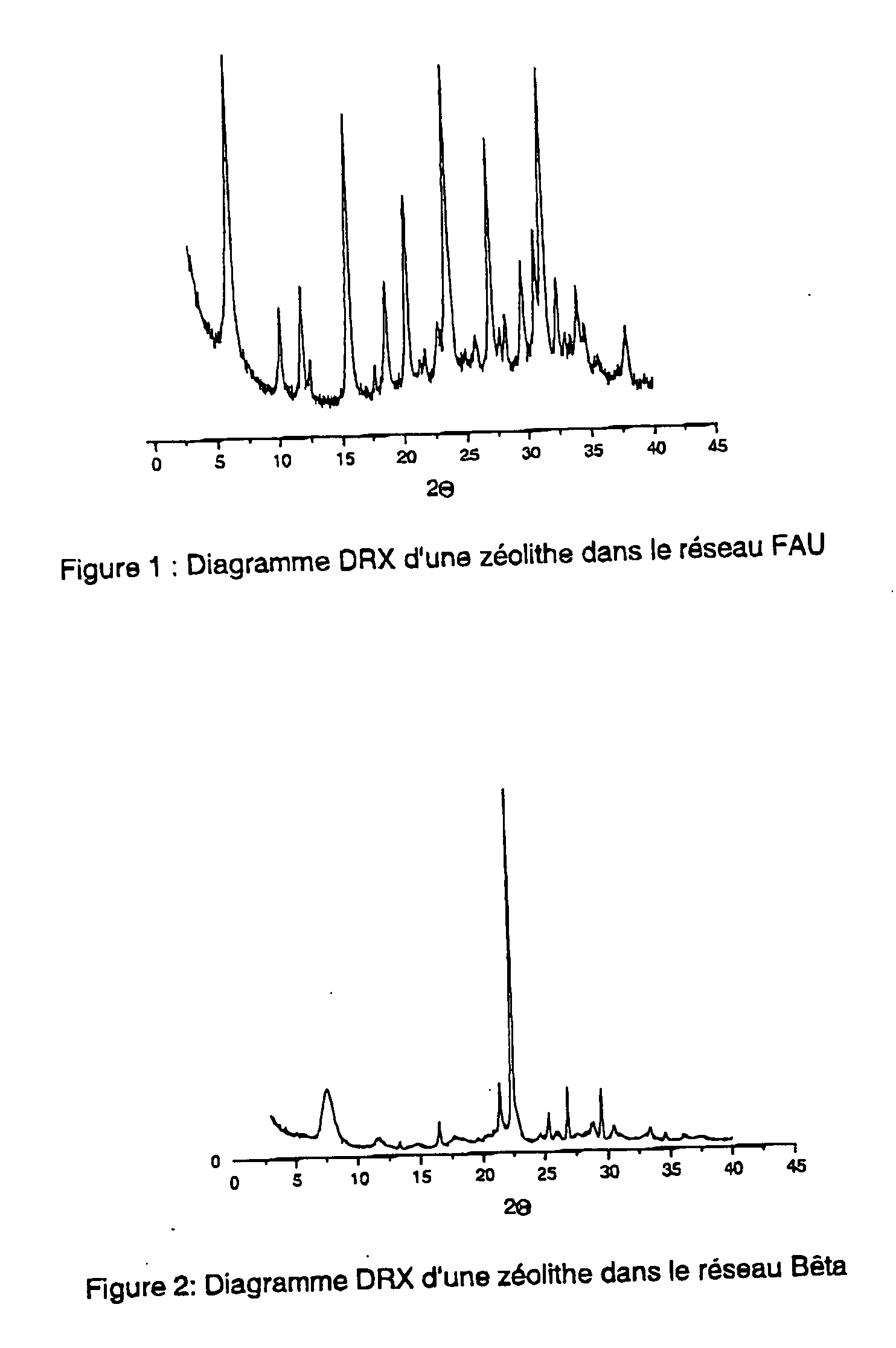
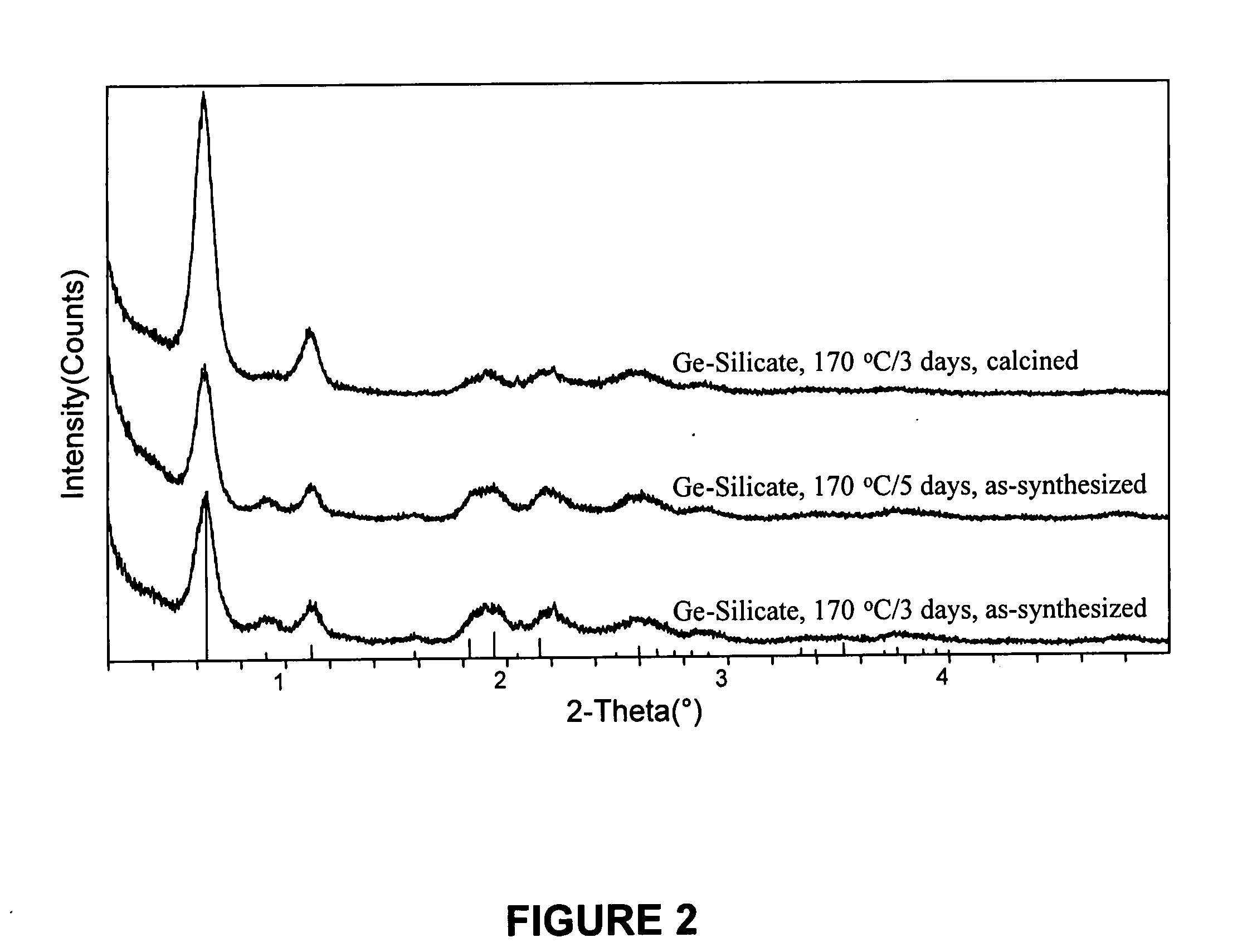
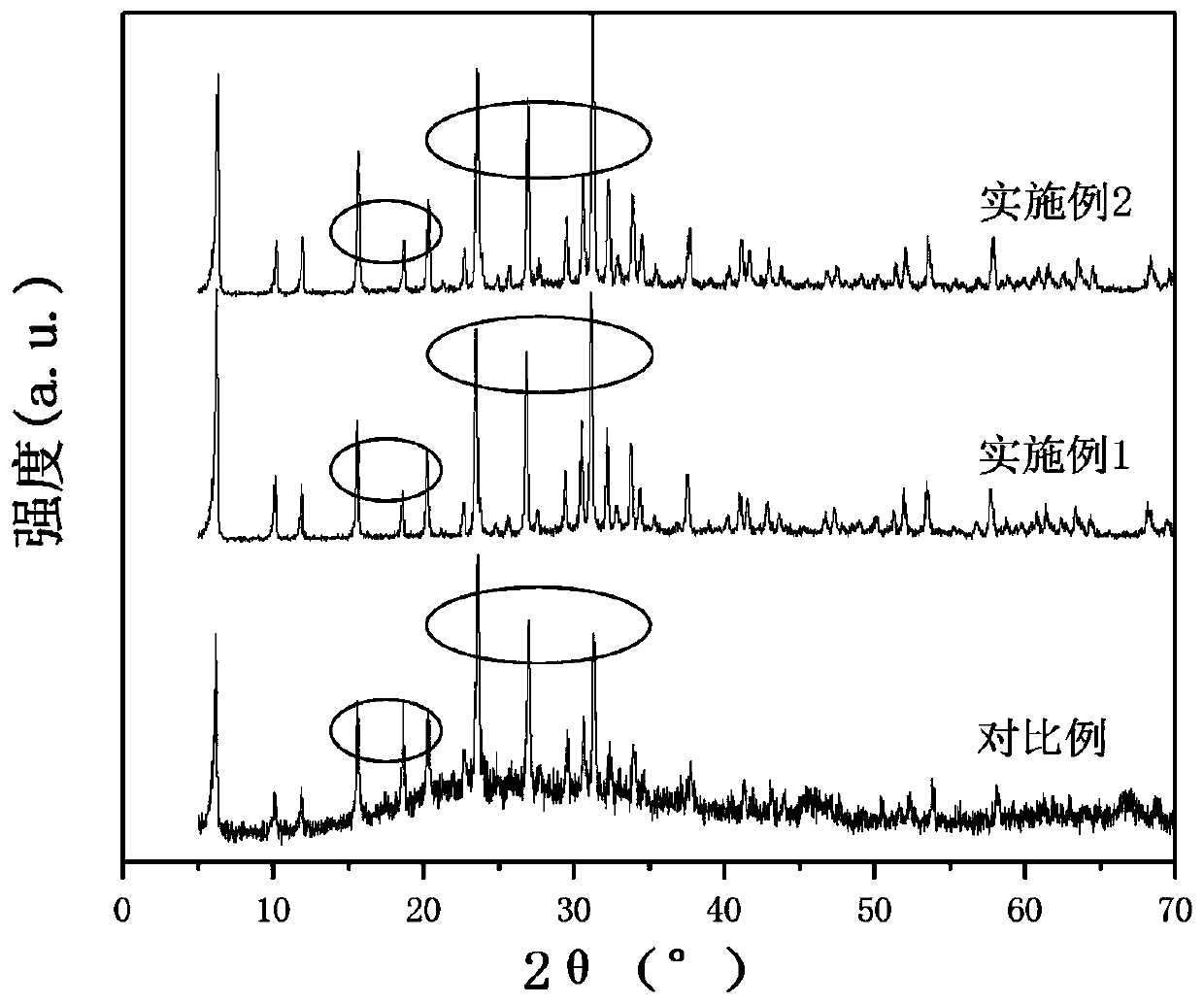
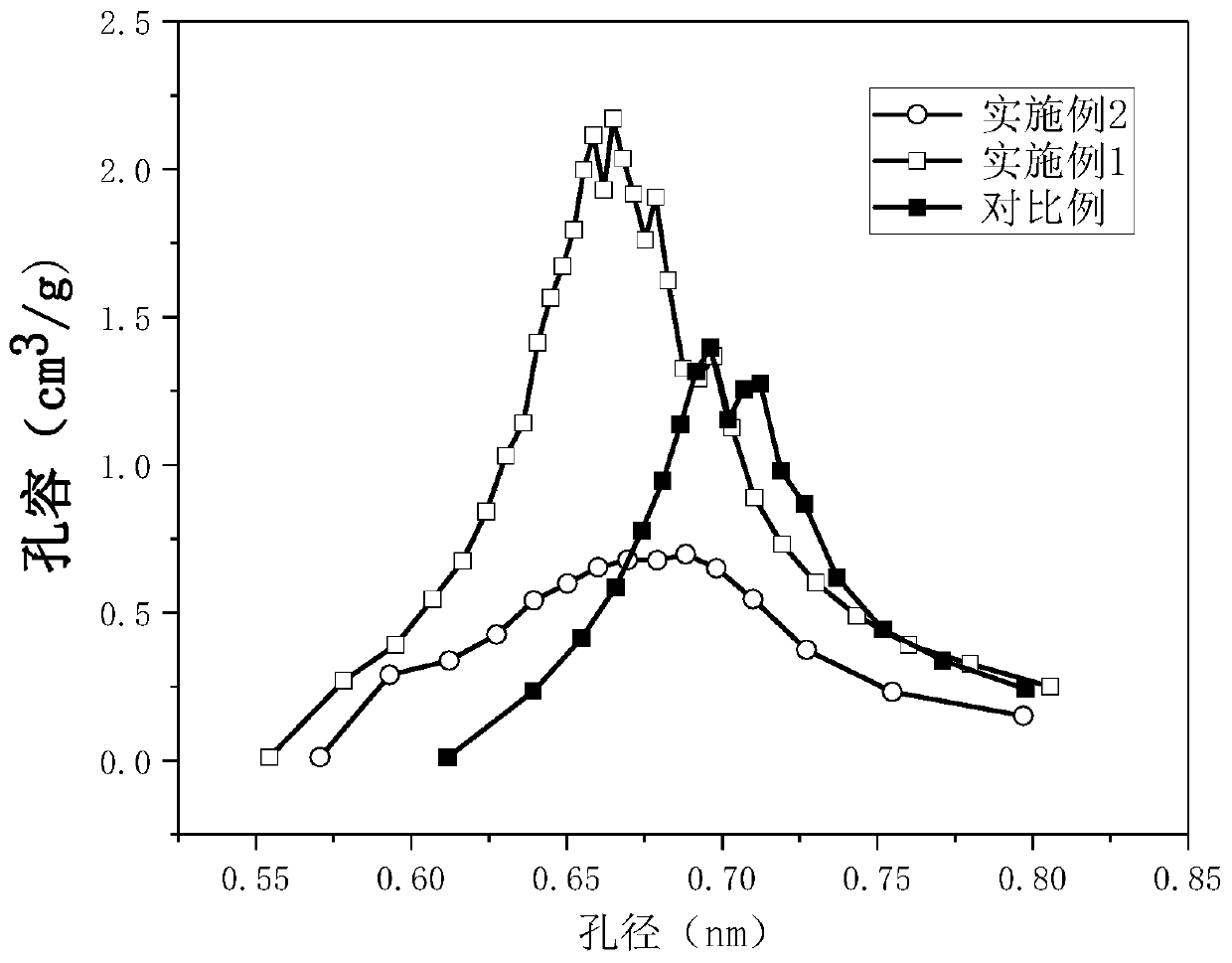
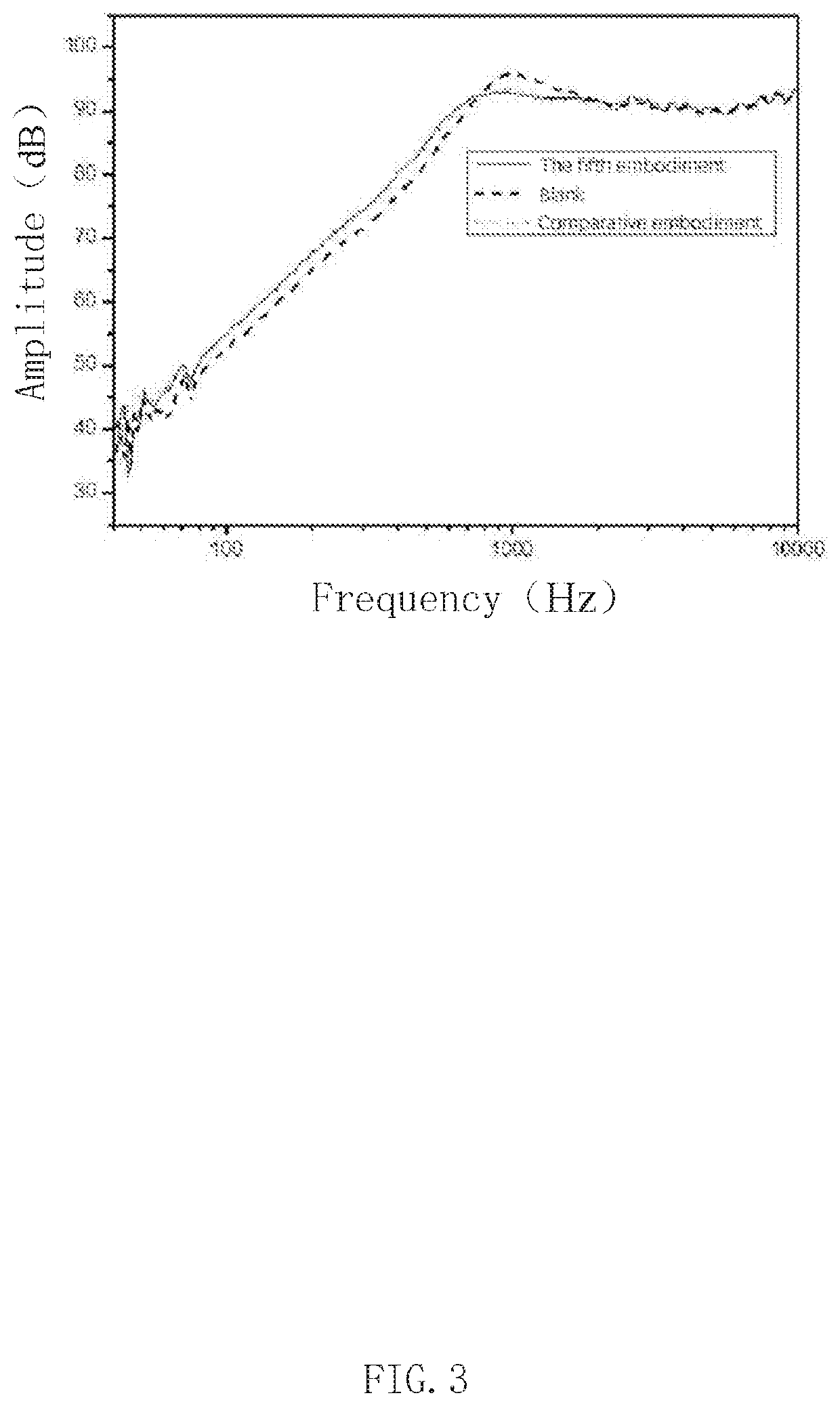
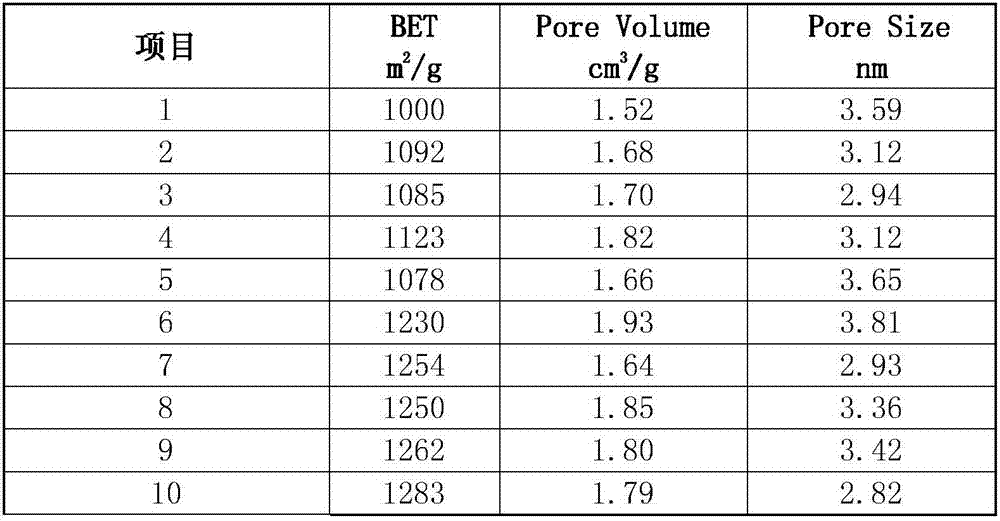
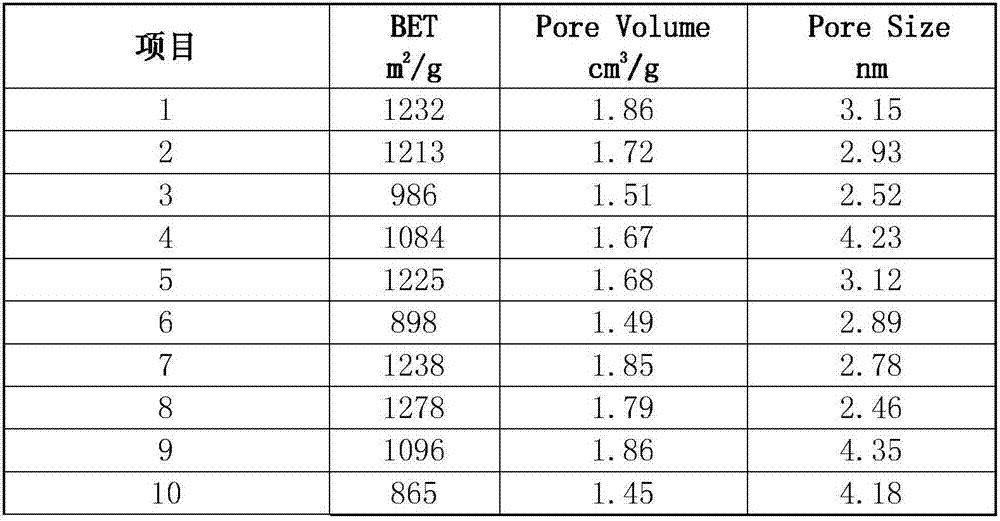
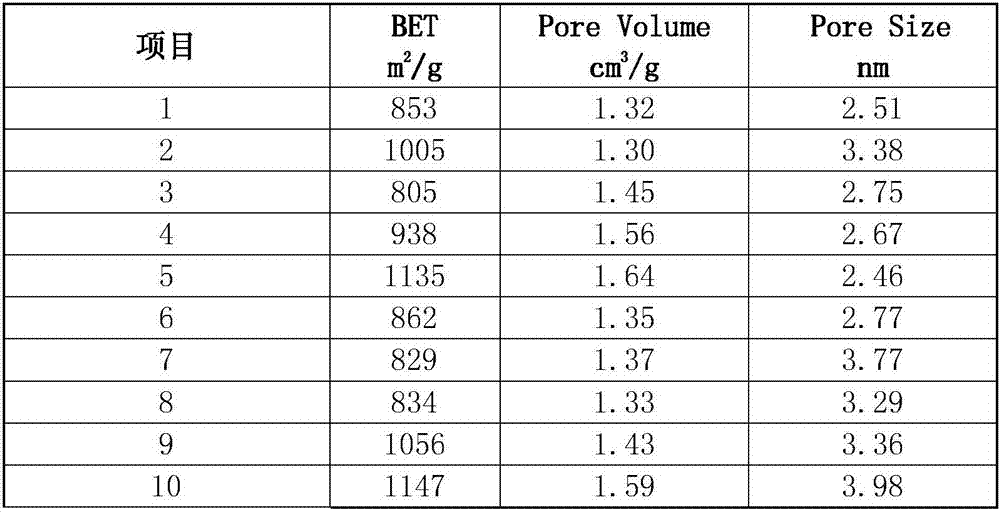
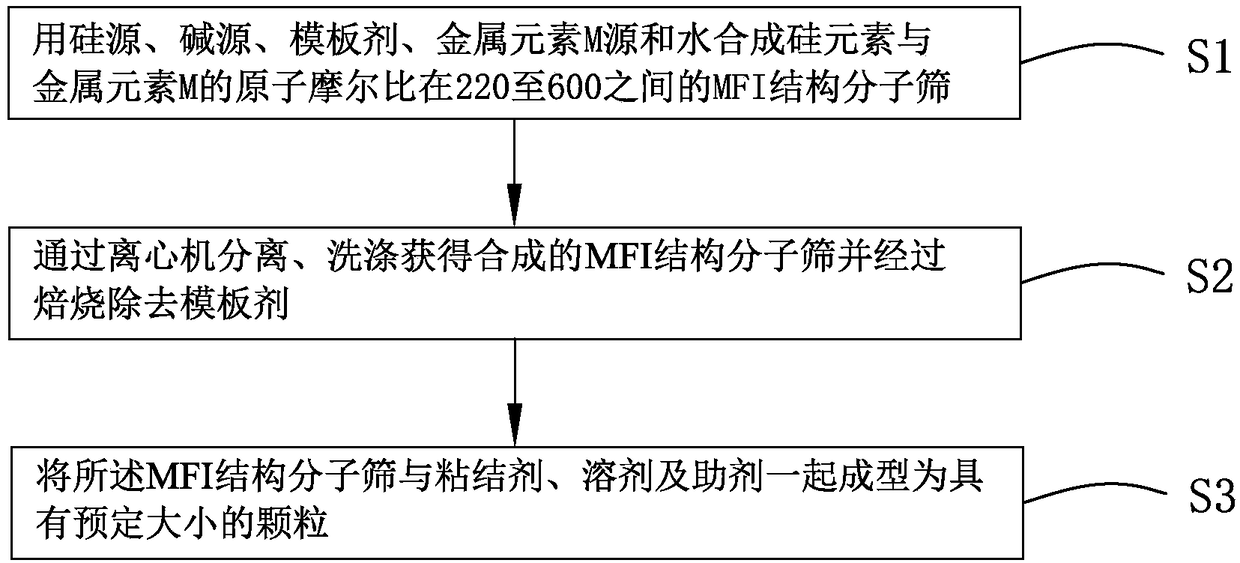
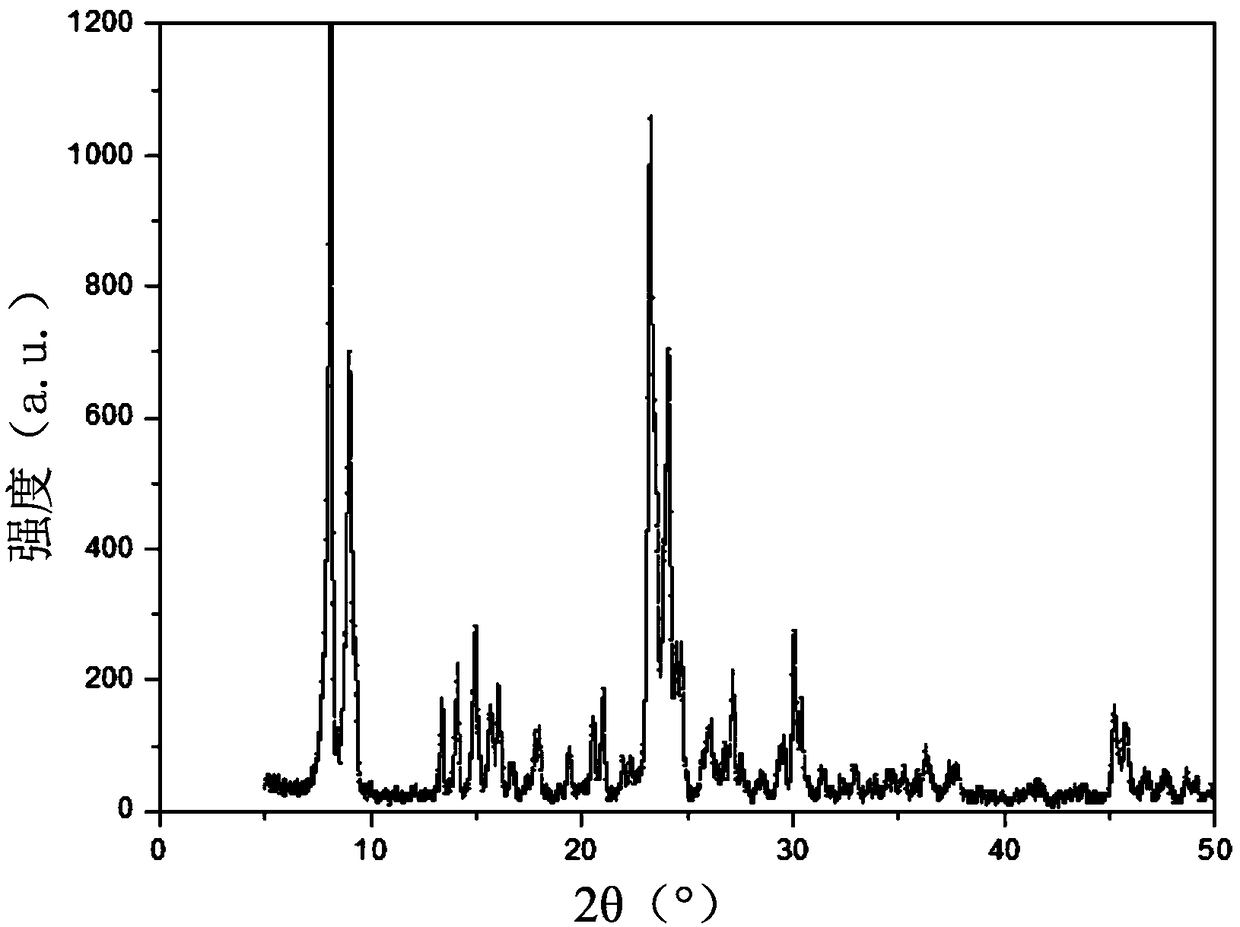
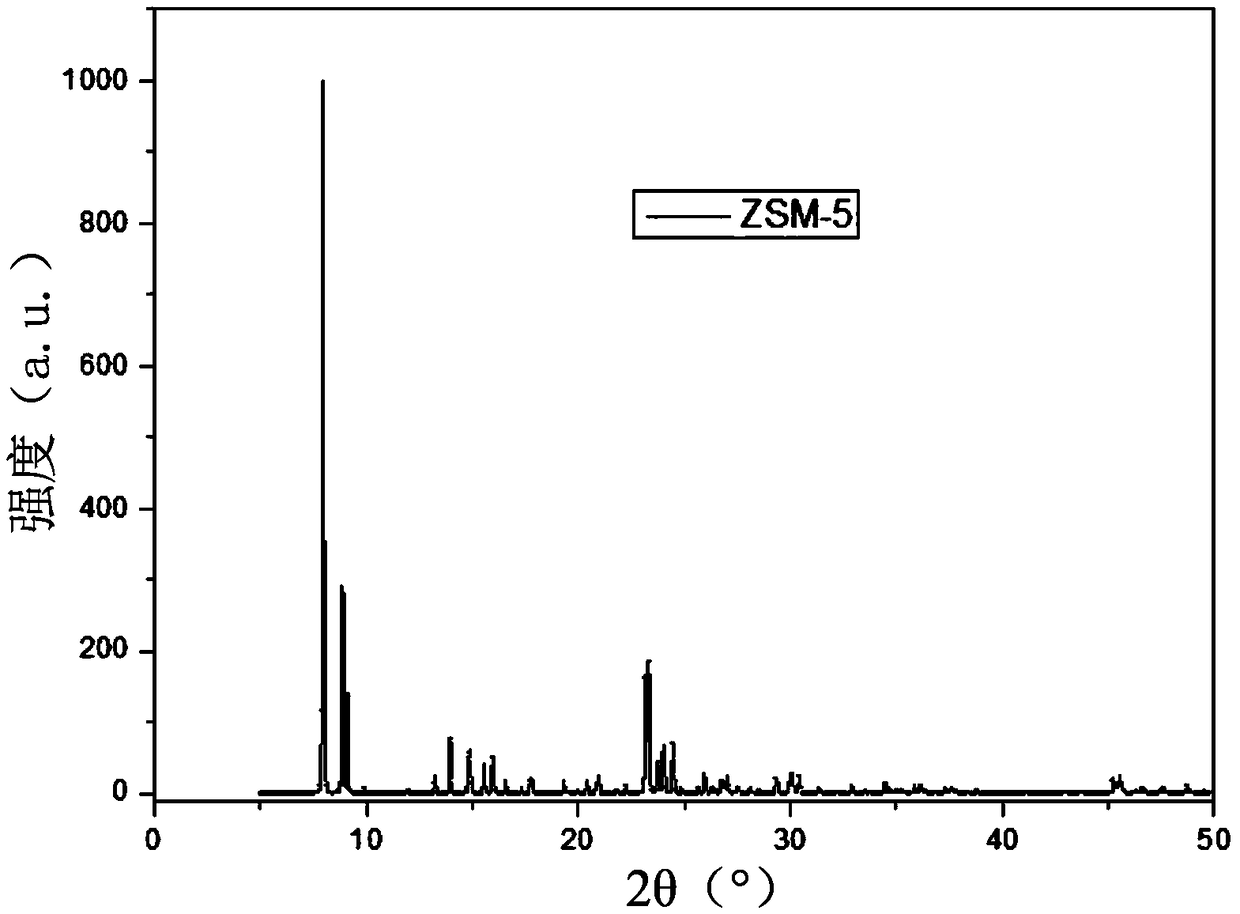
Patents
Literature
91results about "Galloaluminosilicates/ferroaluminosilicates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
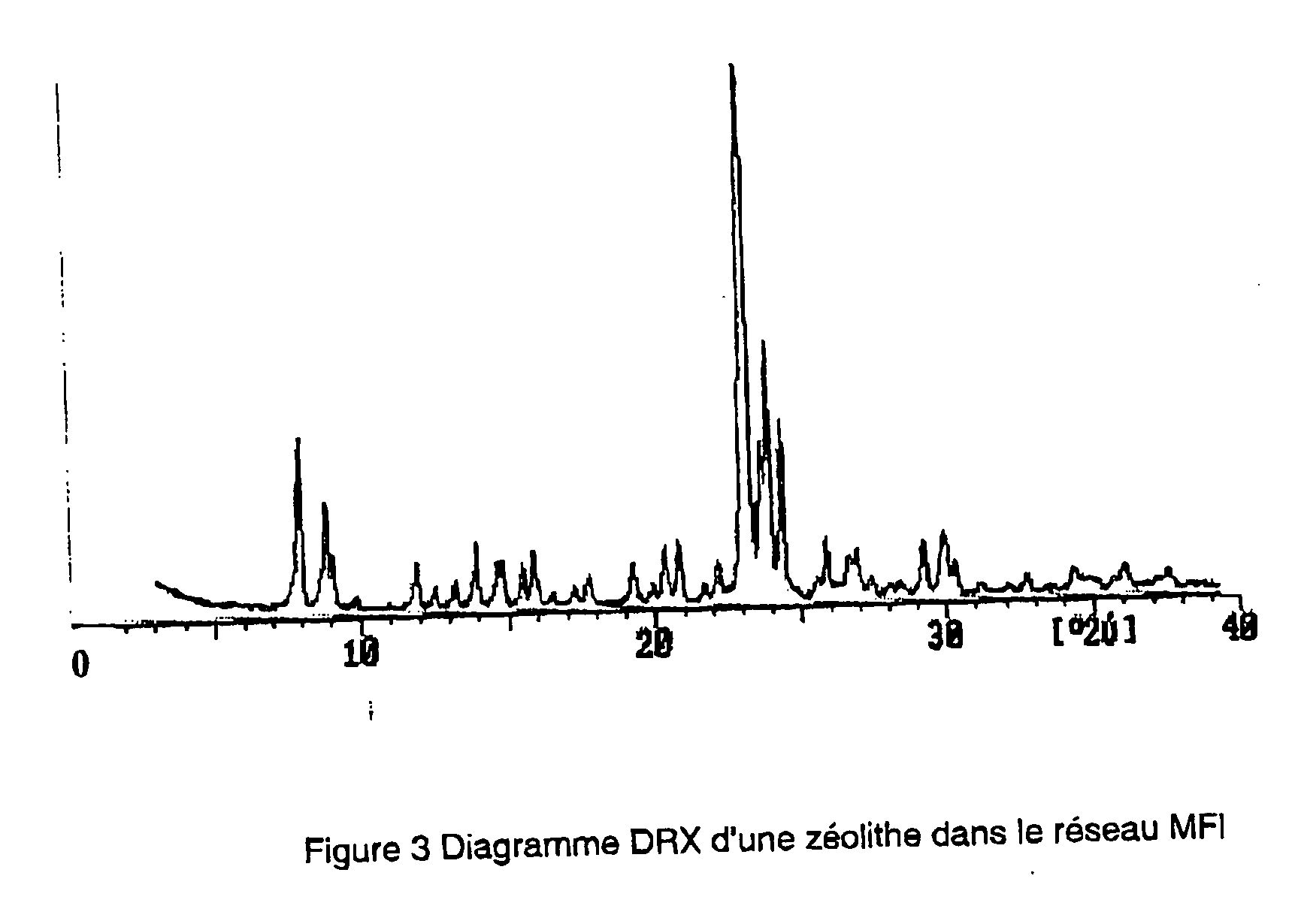
Rapid synthesis of beta zeolites
ActiveUS9108190B1Shorten the timeShorten the compositing timeSugar derivativesMolecular sieve catalystsIsomerizationHeteroatom
The invention provides methods for rapidly synthesizing heteroatom containing zeolites including Sn-Beta, Si-Beta, Ti-Beta, Zr-Beta and Fe-Beta. The methods for synthesizing heteroatom zeolites include using well-crystalline zeolite crystals as seeds and using a fluoride-free, caustic medium in a seeded dry-gel conversion method. The Beta zeolite catalysts made by the methods of the invention catalyze both isomerization and dehydration reactions.
Owner:UNIV OF MASSACHUSETTS
Method for synthesizing a crystalline metalloaluminosilicate by direct synthesis
The invention concerns a method for preparing a crystalline metalloaluminosilicate by direct synthesis using at least one source of aluminium and, as the source of silicon and as the source of at least one other metal M, at least one lamellar siliceous material containing metals in its framework. The invention also concerns the novel solids obtained, in particular solids with a given zeolitic structure containing particular metals in its zeolitic framework.
Owner:INST FR DU PETROLE
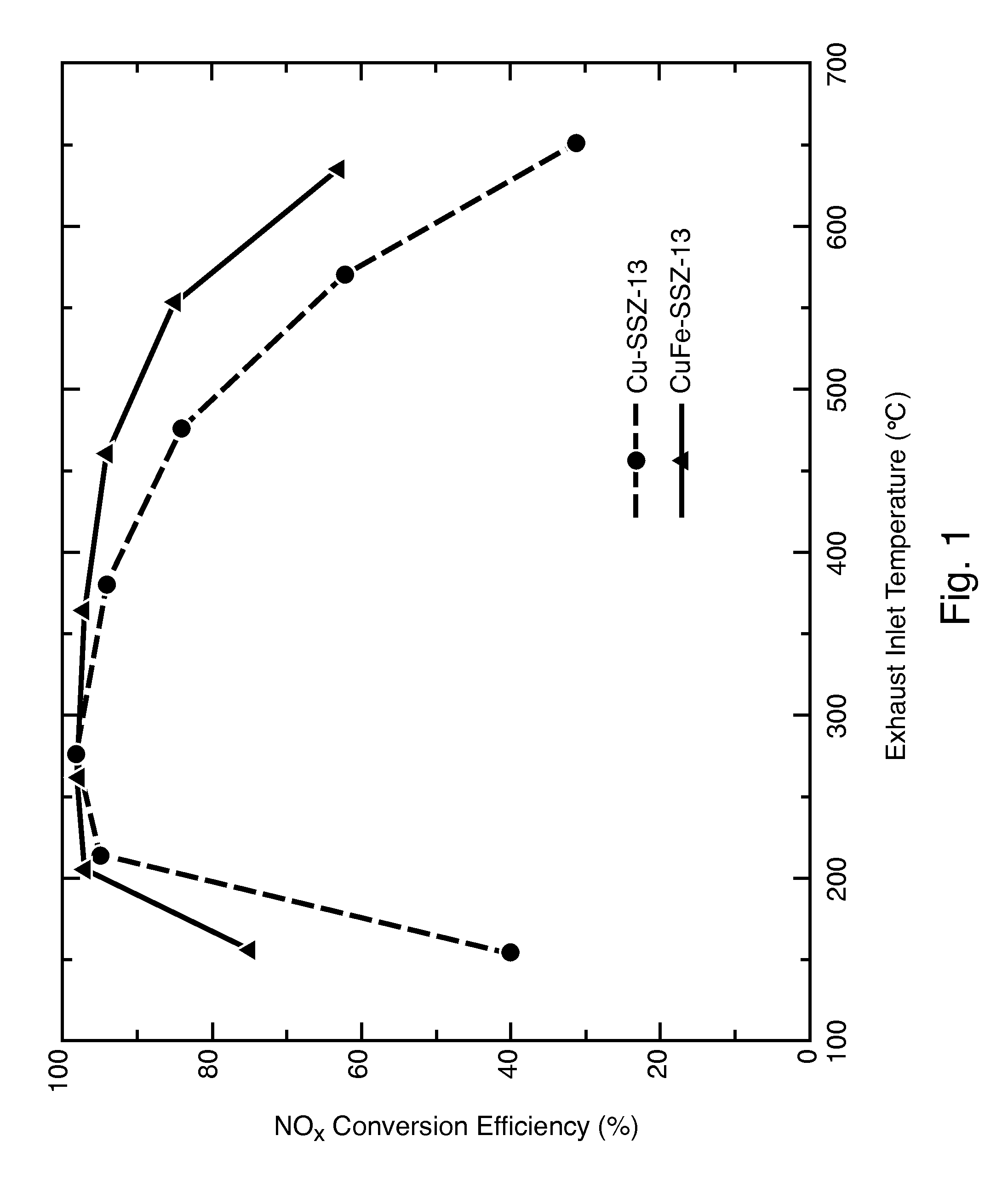
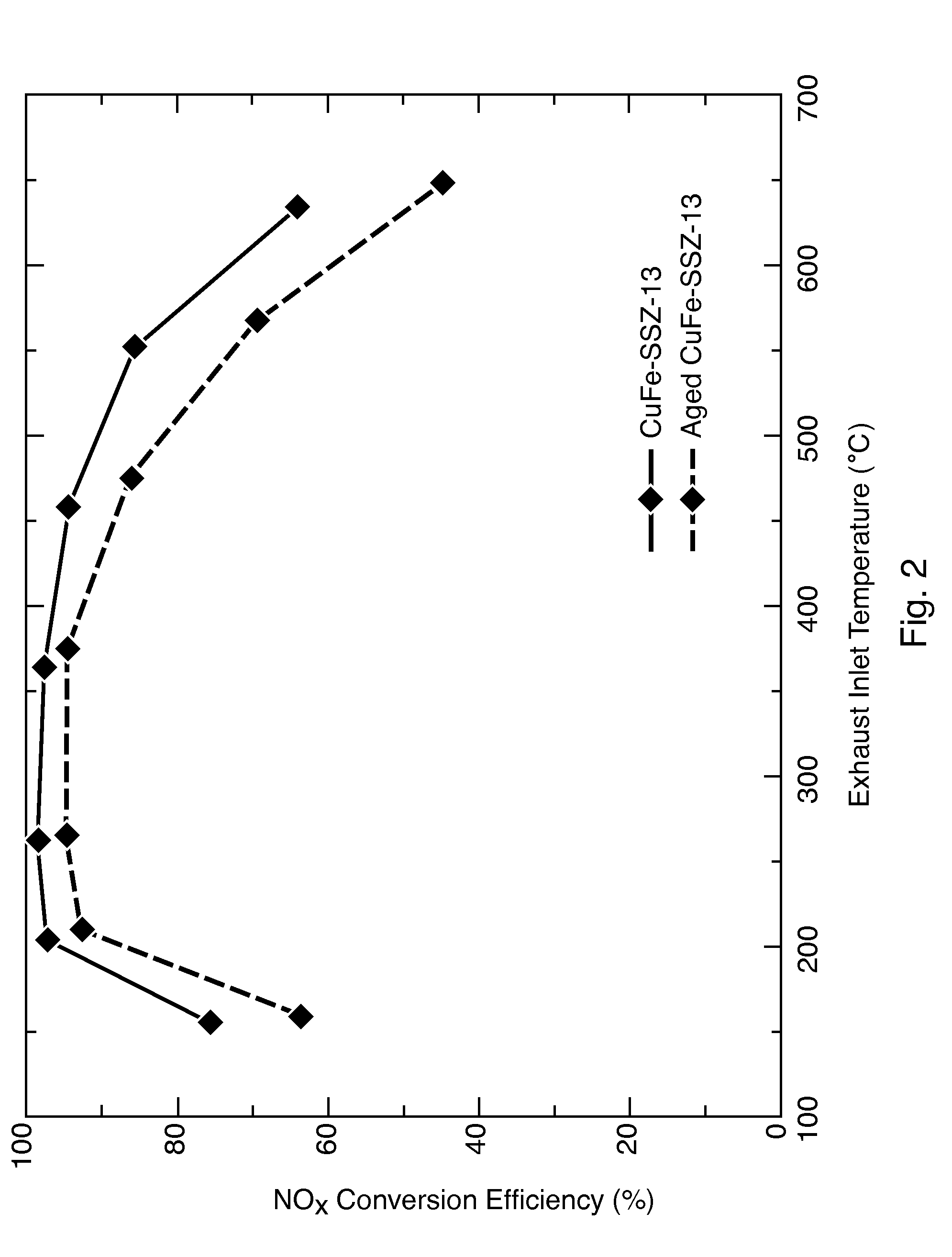
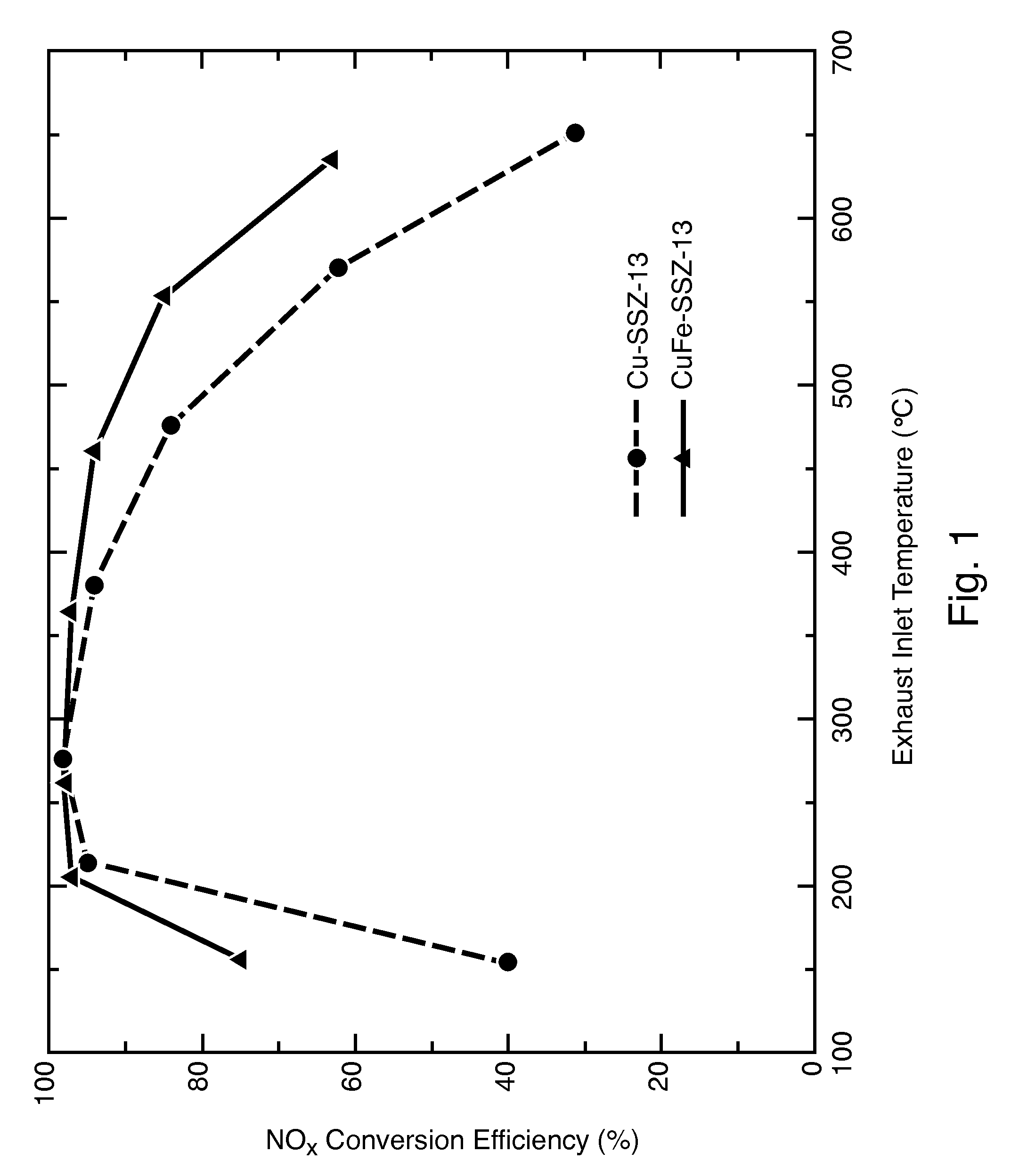
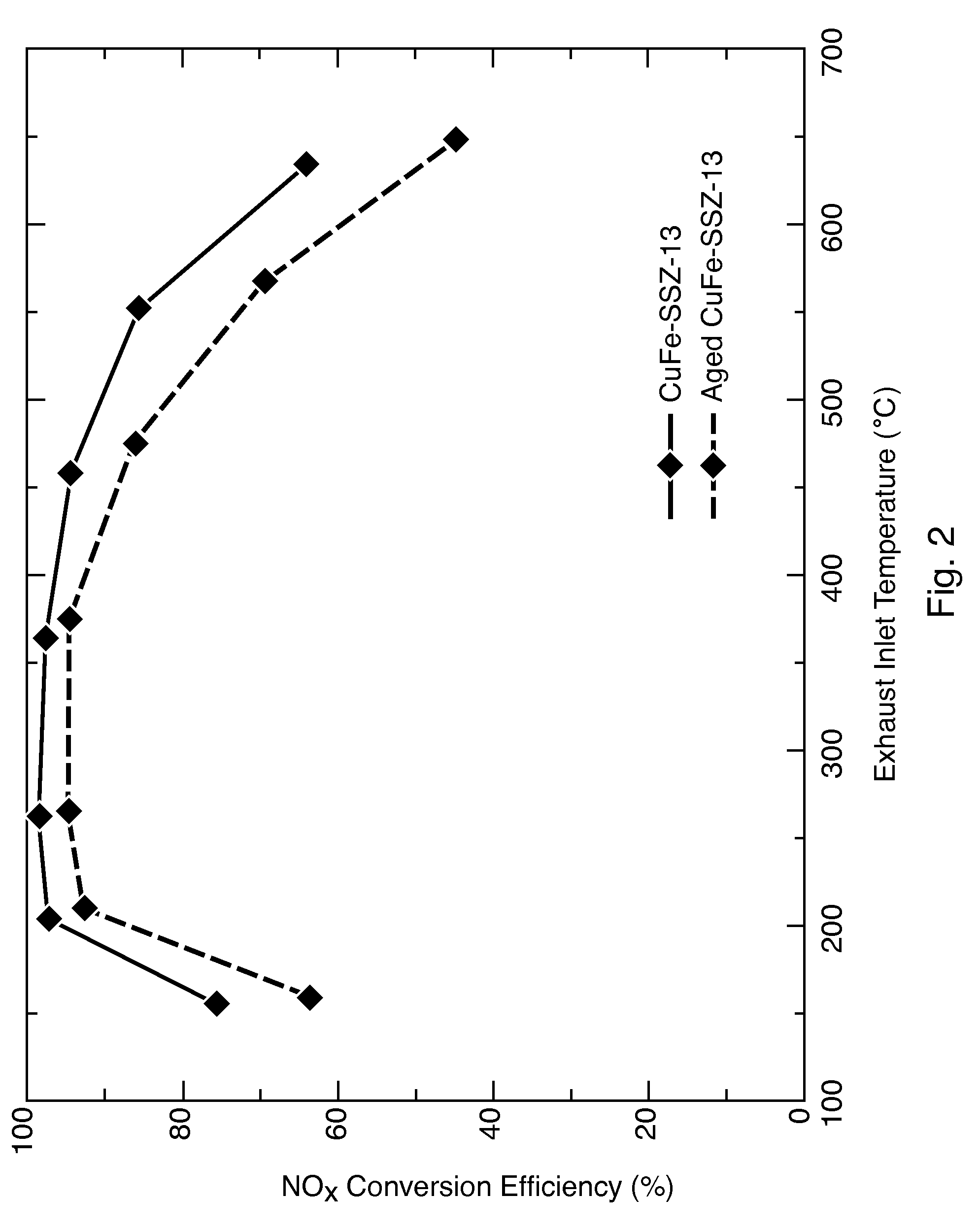
Hydrothermally Stable, Low-Temperature NOx Reduction NH3-SCR Catalyst
ActiveUS20130224082A1Reduce nitrogen oxide emissionsSuitable for operationCombination devicesGas treatmentPhysical chemistryExhaust fumes
A catalyst composition includes a heterobimetallic zeolite characterized by a chabazite structure loaded with copper ions and at least one trivalent metal ion other than Al3+. The catalyst composition decreases NOx emissions in diesel exhaust and is suitable for operation in a catalytic converter.
Owner:UT BATTELLE LLC
Novel methods for producing crystalline microporous solids with the heu topology and compositions derived from the same
ActiveUS20150202603A1Raise the ratioHigh activityCatalytic crackingHydrocarbon oils refiningPhysical chemistryMineralogy
This disclosure relates to new crystalline microporous solids (including silicate- and aluminosilicate-based solids), the compositions comprising 8 and 10 membered inorganic rings, particularly those having HEU topologies having a range of Si:Al ratios, methods of preparing these and known crystalline microporous solids using certain quaternized imidazolium cation structuring agents.
Owner:CALIFORNIA INST OF TECH
Low-cost green method for synthesizing Fe-ZSM-5 molecular sieve
InactiveCN105731492AReduce manufacturing costToxic reductionGalloaluminosilicates/ferroaluminosilicatesPentasil aluminosilicate zeoliteNatural mineralMaceral
The invention belongs to the technical field of molecular sieves and preparation methods thereof, and particularly relates to a technique for preparing an Fe-ZSM-5 molecular sieve. According to the method, natural mineral diatomite is used as the raw material to provide all of the silicon source, aluminum source and iron source required by synthesis of Fe-ZSM-5, and conventional hydrothermal crystallization is carried out under the condition of not adding any organic template. The natural mineral diatomite is used as the raw material to provide all the silicon and aluminum sources required by the synthesis of the molecular sieve, the Fe2O3 impurity contained in the diatomite raw material is fully utilized as the iron source, and a conventional hydrothermal process is carried out under the condition of not adding any organic template, thereby preparing the framework-iron-containing ZSM-5 molecular sieve (Fe-ZSM-5 molecular sieve). The method has obvious advantages in the aspects of widening the molecular sieve synthesis raw material range, enhancing the added value of the natural mineral product, lowering the molecular sieve production cost, reducing the environmental pollution and the like.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Aluminosilicate compositions, preparation and use
A metalloaluminosilicate composition includes an aluminosilicate composition having an aluminosilicate framework and containing at least one metal, wherein a substantial portion of the metal is incorporated into the aluminosilicate framework. A higher concentration of the metal is incorporated into the framework of the catalyst than is present at the surface of the catalyst.
Owner:INTREVEP SA
One-step method used for synthesis of iron-based molecular sieve catalyst, and applications thereof
ActiveCN109985660AEvenly distributedSimple processNitrous oxide captureGas treatmentIron saltsFiltration
The invention provides a one-step method used for synthesis of an iron-based molecular sieve catalyst. The one-step method comprises following steps: a Fe-complex solution and an aluminosilicate molecular sieve gel system are prepared respectively; the Fe-complex solution is added into the aluminosilicate molecular sieve gel system slowly, full stirring is carried out, an obtained mixture is allowed to stand for aging, is introduced into a reaction kettle for crystallization, is washed with deionized water, and is subjected to pumping filtration, drying, and roasting so as to obtain a solid iron-based molecular sieve; and then ammonium exchange is carried out so as to obtain the iron-based molecular sieve catalyst. The technology of the one-step method is simple; adoption of a plurality oftimes of ammonium nitrate and iron salt solution ion exchange and calcining is avoided, a defect in the prior art that later stage ion exchange technology is needed for loading of the active component is avoided, the synthesized iron-based molecular sieve catalyst is better in catalytic activity, Fe distribution is more uniform, excellent NH3-SCR catalytic activity is maintained in a wider temperature window, and at the same time, excellent high temperature activity and N2 selectivity are achieved.
Owner:HUAZHONG UNIV OF SCI & TECH
IRON-ZEOLITE CHABAZITE CATALYST FOR USE IN NOx REDUCTION AND METHOD OF MAKING
InactiveUS20150231620A1Good high-temperature activityReduction of nitrogen oxidesCombination devicesAluminium compoundsNitrogen oxidesIon exchange
An iron-zeolite chabazite (CHA) catalyst is provided as an SCR catalyst for reducing nitrogen oxides (NOx) from vehicle engine exhausts. The catalyst is formed by incorporating iron during synthesis of the chabazite zeolite, which eliminates the need for a post-synthesis ion-exchange step and which results in the incorporation of iron into the (CHA) zeolite crystal lattice structure. The resulting catalyst exhibits good high temperature activity at temperatures greater than 550° C. and exhibits good thermal stability.
Owner:FORD GLOBAL TECH LLC
Process for preparing crystalline microporous and mesoporous metal silicates, products obtainable by said process and their use
Microporous and mesoporous metal silicates are prepared by the hydrothermal reaction of a silicon and metal source in the presence of a template. The choice of raw materials influences the purity and hence the catalytic activity of the products. A pyrogenic mixed oxide is used as the silicon and metal source. Prepared products have the composition (SiO2)1-x(AmOn)x, where A is Ti, Al, B, V or Zr and x is 0.005 to 0.1. Shaped objects of the microporous and mesoporous metal silicates are obtained directly by using a shaped object of the pyrogenic mixed oxide. The products obtained are used as oxidation catalysts.
Owner:EVONIK DEGUSSA GMBH
Process for preparing crystalline microporous and mesoporous metal silicates, products obtainable by said process and their use
Owner:EVONIK DEGUSSA GMBH
High activity MTT framework type molecular sieves
ActiveUS20100147741A1Aluminium compoundsMolecular sieve catalystsMolecular sieveAlkaline earth metal
A process is described of producing an MTT framework type molecular sieve by crystallizing a mixture capable of forming said molecular sieve, wherein the mixture comprises sources of alkali or alkaline earth metal (M), an oxide of trivalent element (X), an oxide of tetravalent element (Y), water and a directing agent (R) of the formula (CH3)3N+CH2CH2CH2N+(CH3)2CH2CH2CH2N+(CH3)3, and has a composition, in terms of mole ratios, within the following ranges.YO2 / X2O3 less than 45H2O / YO2 5 to 100OH− / YO2 0.05 to 0.5M / YO2 0.05 to 0.5 andR / YO2>0 to <0.5.
Owner:EXXON RES & ENG CO
Hydrothermally stable, low-temperature NOx reduction NH3-SCR catalyst
A catalyst composition includes a heterobimetallic zeolite characterized by a chabazite structure loaded with copper ions and at least one trivalent metal ion other than Al3+. The catalyst composition decreases NOx emissions in diesel exhaust and is suitable for operation in a catalytic converter.
Owner:UT BATTELLE LLC
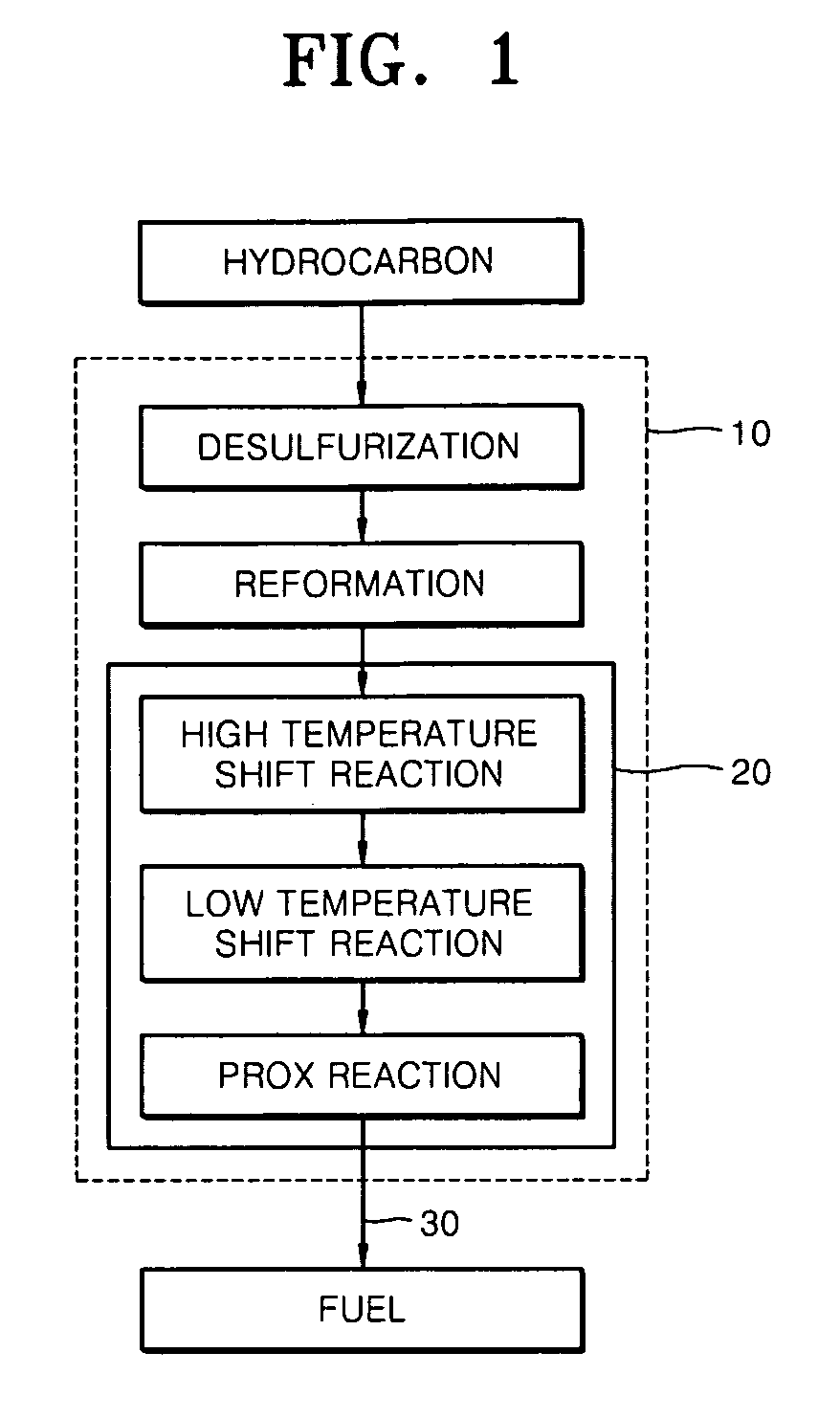
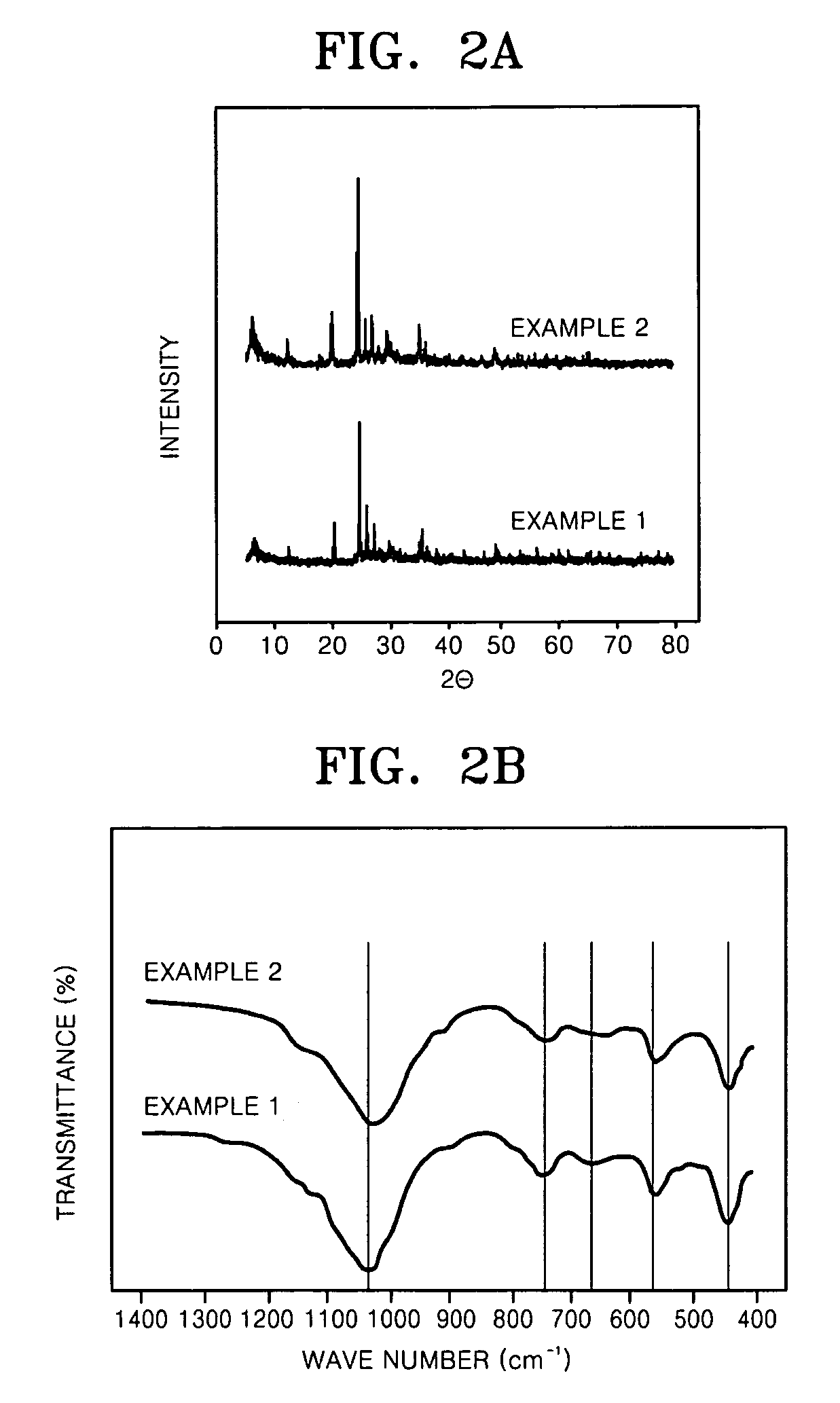
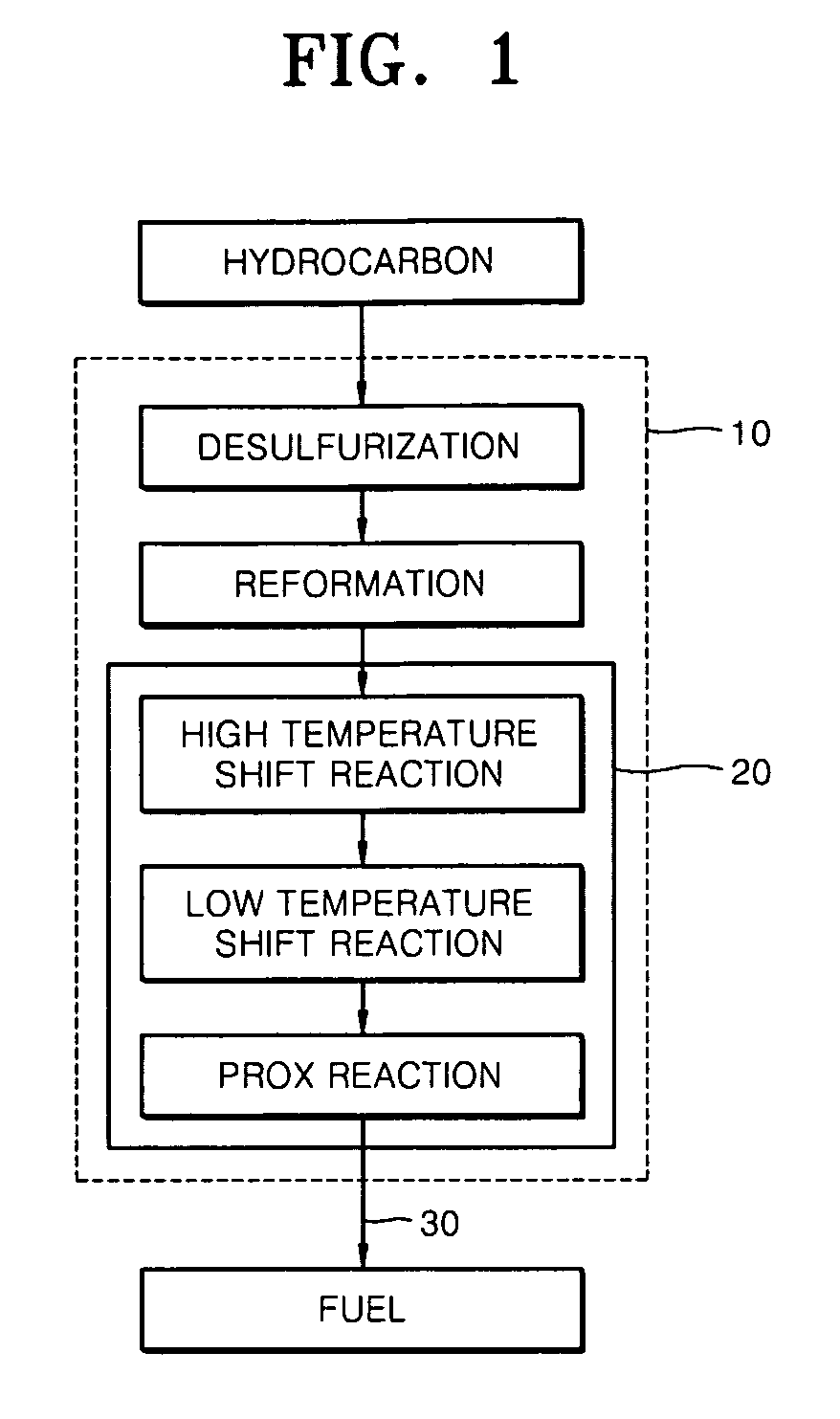
Desulfurization adsorbent for fuel cell and desulfurizing method using the same
InactiveUS20070093385A1Improve desulfurization performancePromote regenerationMethane captureMolecular-sieve silicatesAlkaline earth metalSorbent
A desulfurization adsorbent for a fuel cell has a structure according to Formula 1 below, and a desulfurizing method uses the desulfurization adsorbent. The desulfurization adsorbent displays remarkably excellent adsorption performance for adsorbing sulfur compounds as well as excellent regeneration performance, compared with conventional desulfurization adsorbents. Thus, the desulfurization adsorbent does not need to be replaced even after prolonged use, thus stabilizing the operation of a fuel cell system and reducing costs. (M1)a-(Si)x—(Ti)y-(M2)z-O [Formula 1]wherein M1 is at least one selected from alkali metals, alkaline earth metals, hydrogen, ammonium, rare earths, and transition metals; 4≦x / y≦500, 0≦z / y≦3, 0≦a / (y+z)≦1; and M2 is aluminum (Al), boron (B) or a trivalent metal. The desulfurizastion adsorbent is produced by subjecting a mixture of a silicon source, a titanium source, and optionally, aluminum, boron or a trivalent metal in an alkali solution to a hydrothermal treatment to obtain a crystalline porous molecular sieve.
Owner:SAMSUNG SDI CO LTD
Molecular sieve having mesopores, preparation method therefor, and application thereof
ActiveUS20200325029A1Increased proportion of volumeHigh catalytic efficiencyHydrocarbon by isomerisationMolecular sieve catalystsMolecular sieveIsomerization
A molecular sieve has a silica / alumina molar ratio of 100-300, and has a mesopore structure. One closed hysteresis loop appears in the range of P / P0=0.4-0.99 in the low temperature nitrogen gas adsorption-desorption curve, and the starting location of the closed hysteresis loop is in the range of P / P0=0.4-0.7. The catalyst formed from the molecular sieve as a solid acid not only has a good capacity of isomerization to reduce the freezing point, but also can produce a high yield of the product with a lower pour point. The process for preparing the catalyst involves steps including crystallization, filtration, calcination, and hydrothermal treatment.
Owner:CHINA PETROCHEMICAL CORP +1
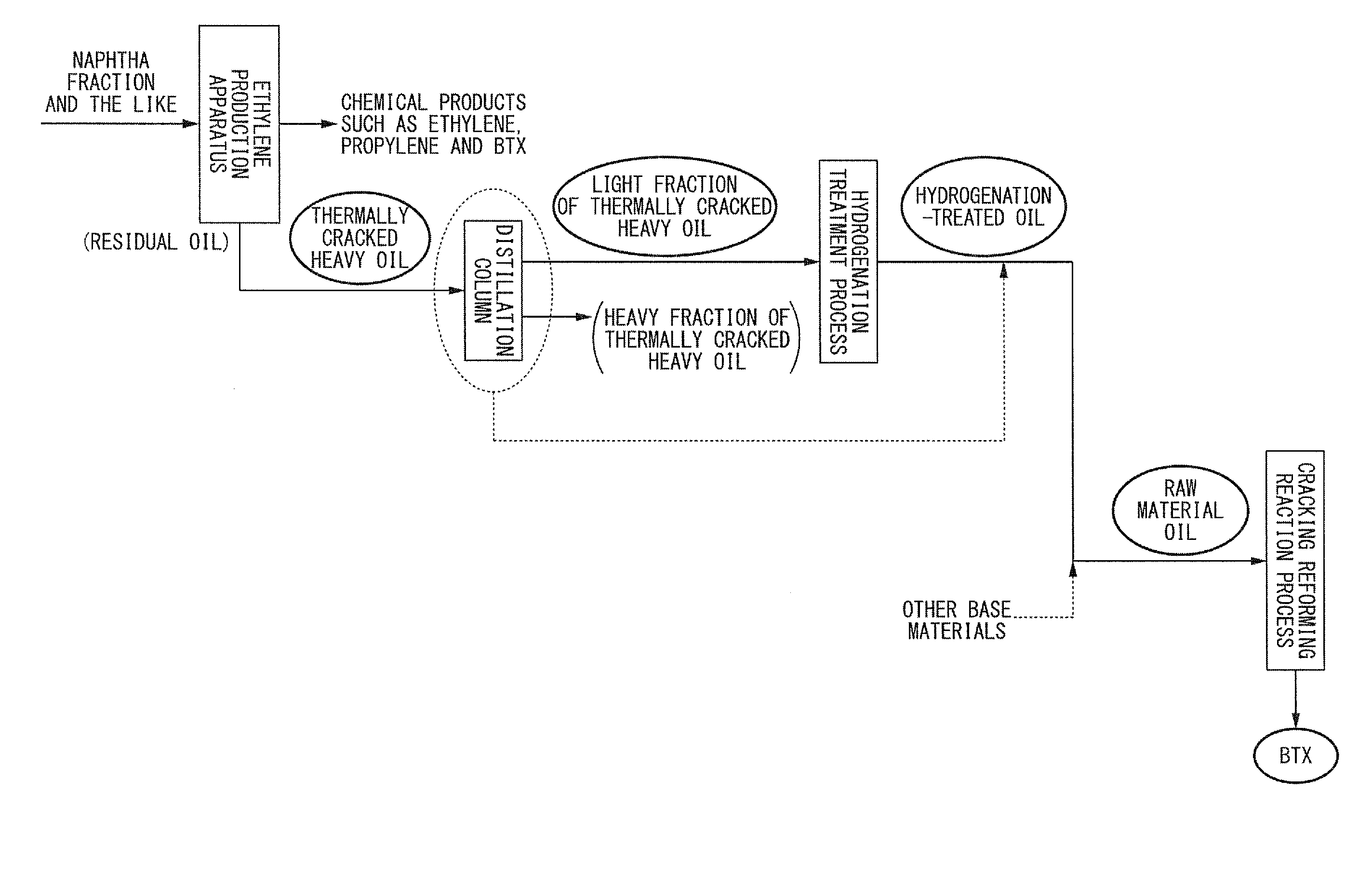
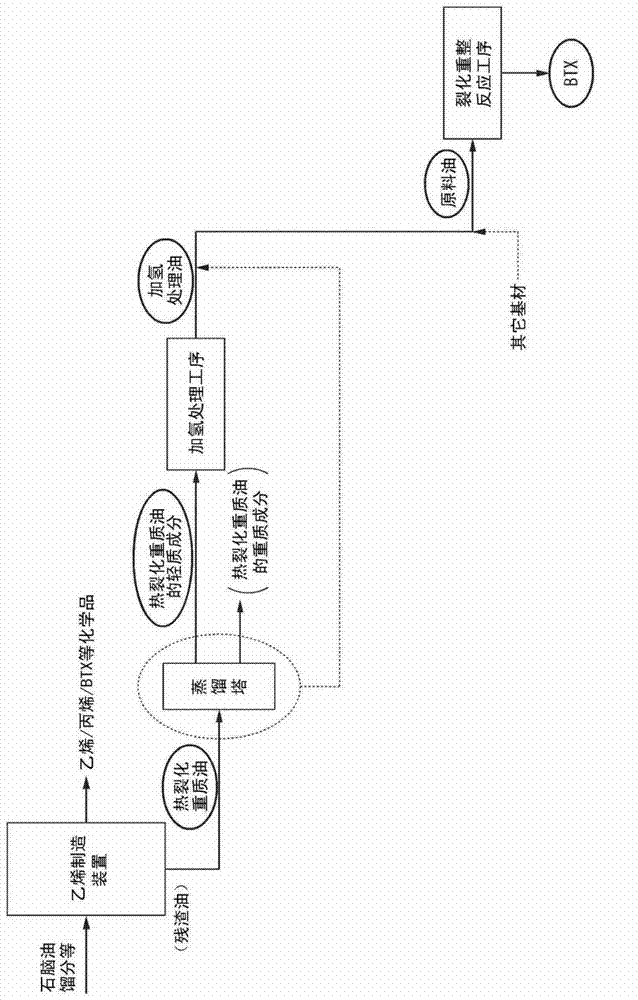
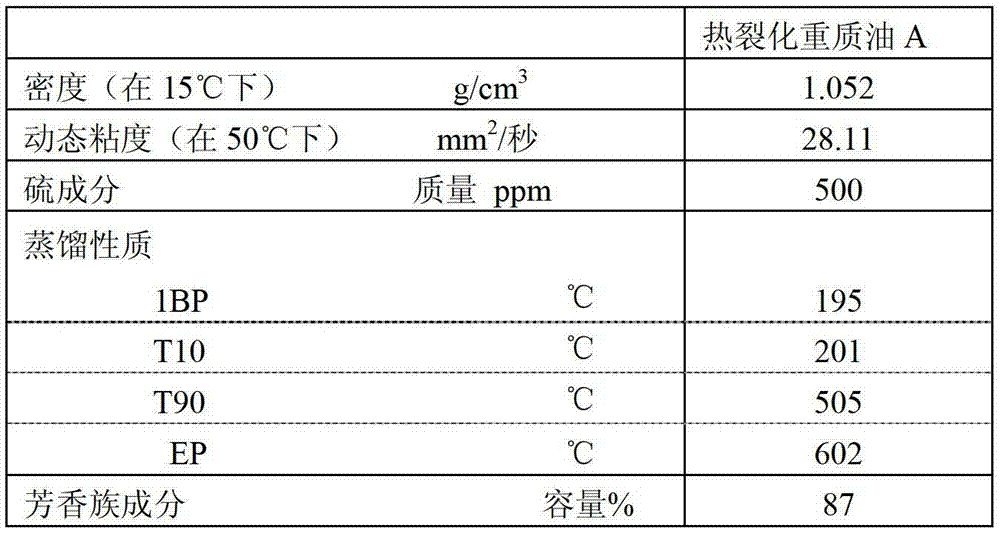
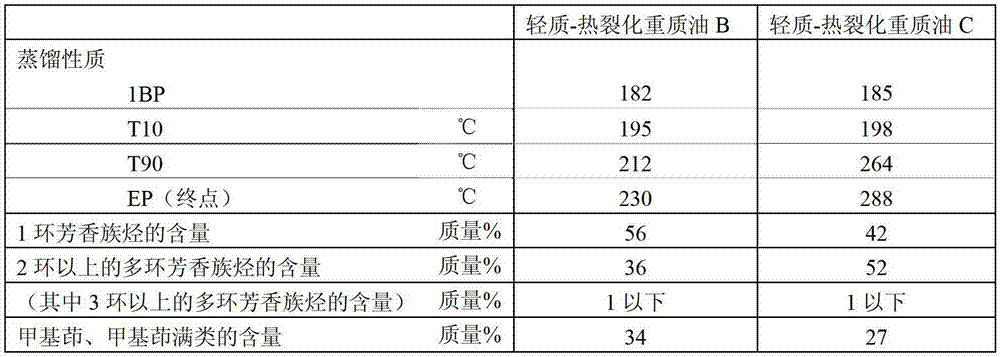
Method for producing aromatic hydrocarbons
ActiveUS20130172639A1Efficient productionThermal non-catalytic crackingTreatment with hydrotreatment processesDistillationFuel oil
Provided is a method for producing aromatic hydrocarbons, by which a feedstock containing a hydrogenation-treated oil of a thermally cracked heavy oil obtainable from an ethylene production apparatus is brought into contact with a catalyst for monocyclic aromatic hydrocarbon production containing a crystalline aluminosilicate, and thereby aromatic hydrocarbons are produced. A raw material having an end point of the distillation characteristics of 400° C. or lower is used as the feedstock. The contact between the feedstock and the catalyst for monocyclic aromatic hydrocarbon production is carried out at a pressure of 0.1 MPaG to 1.5 MPaG.
Owner:JX NIPPON OIL & ENERGY CORP
Sound absorption material and loudspeaker box applying sound absorption material
PendingCN108996515AExcellent oxygen adsorption capacityImprove hydrophobicityFerrierite aluminosilicate zeoliteMolecular sieve catalystsMolecular sieveAlkaline earth metal
The invention provides a sound absorption material. The sound absorption material comprises a heteroatom zeolite molecular sieve; the heteroatom zeolite molecular sieve comprises a framework and extra-framework cations; the framework comprises SiO2 and a metal oxide MxOy containing metal M, wherein the molar ratio of Si / M in the framework is 250-500, and the M comprises Fe; and the extra-frameworkcations are at least one selected from a group comprising cuprous ions, monovalent silver ions, monovalent gold ions, alkali metal ions or alkaline earth metal ions. The invention further provides aloudspeaker box applying the sound absorption material. Compared with the related art, the loudspeaker box has good acoustic low-frequency performance.
Owner:AAC TECH NANJING
Method of making porous crystalline materials
The present invention relates to new methods of making crystalline materials, as well as to new crystalline materials obtainable by such methods, and their use in hydrocarbon conversion processes. In one of its aspects, the invention relates to a method for preparing a crystalline molecular sieve comprising oxides of one or more tetravalent element(s), optionally one or more trivalent element(s), and optionally one or more pentavalent element(s), said method comprising submitting to crystallization conditions one or more sources of said oxides in the presence of at least one organic templating agent R of formula C1C2R1R2N+ A− (I), in whichC1 and C2 each independently represent a substituted or unsubstituted cyclohexyl or cyclopentyl group,R1 and R2 each independently represent a methyl group, an ethyl group or a propyl group, or R1 and R2 together with the nitrogen atom they are connected to form a ring containing 5 or 6 atoms, andA represents hydroxyl, fluorine, chlorine, bromine or iodine.
Owner:EXXON RES & ENG CO
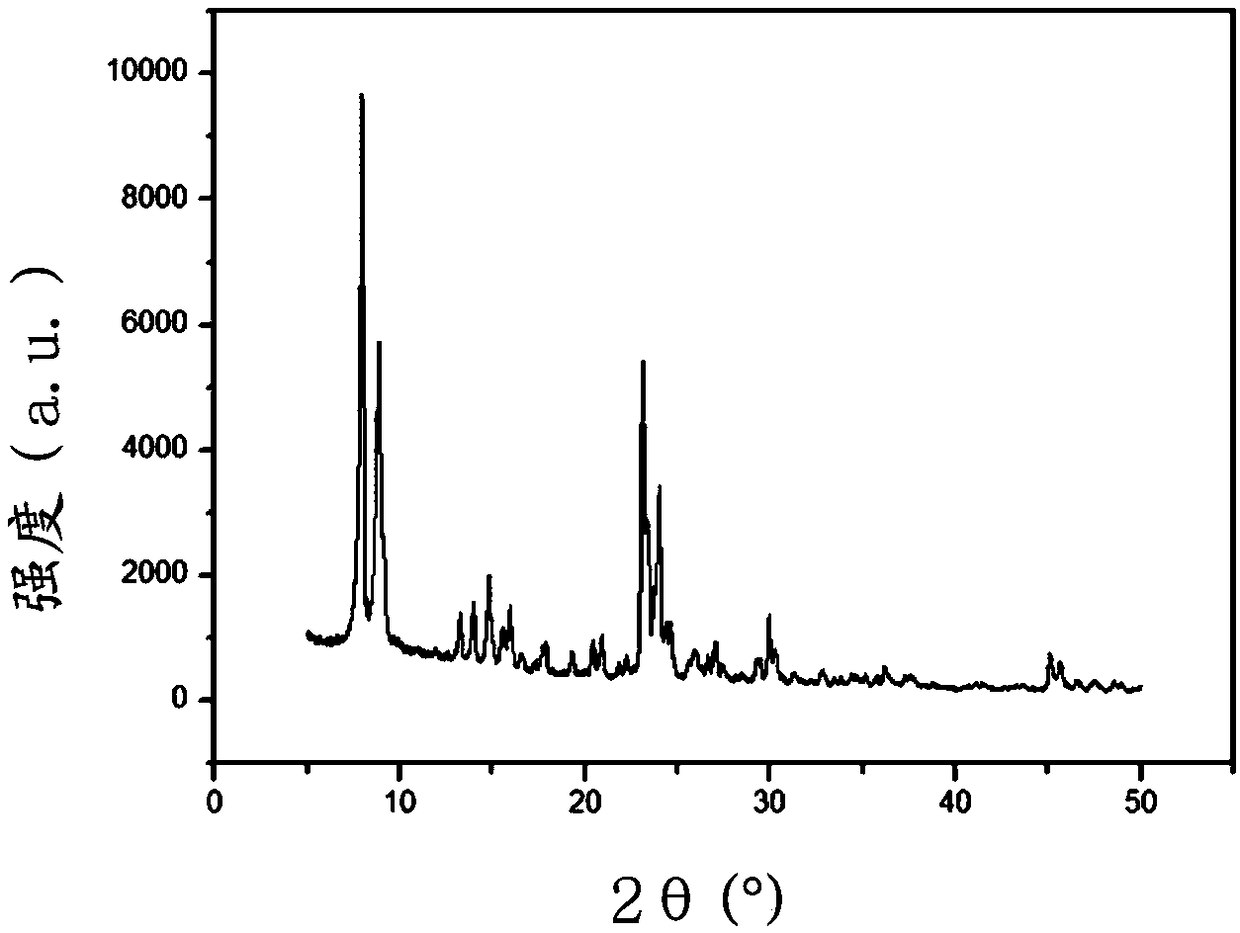
Y molecular sieve for synthesizing dimethyl carbonate through carbonylation of methyl nitrite, and preparation method thereof
ActiveCN110844918AImprove conversion rateHigh space-time yieldCatalyst carriersMolecular sieve catalystsMolecular sievePtru catalyst
The invention discloses a Y molecular sieve for synthesizing dimethyl carbonate through carbonylation of methyl nitrite, and a preparation method thereof, wherein the Y molecular sieve is synthesizedby adopting a hydrothermal method and a microwave-assisted crystallization method. The preparation method comprises: preparing a guiding agent, regulating a mother liquor composition by using a titanium source and a phosphorus source, carrying out microwave pre-crystallization treatment, and carrying out a crystallization reaction to obtain the Y molecular sieve. According to the invention, the Ymolecular sieve is characterized in that titanium and phosphorus heteroatoms are doped into a framework on the basis of keeping of a Y molecular sieve framework structure, and the active component loading molecular sieve is used as a chlorine-free system catalyst for synthesizing dimethyl carbonate through carbonylation of methyl nitrite to achieve good catalytic activity.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Zeolite catalysts, methods for producing zeolite catalysts, and methods for producing lower olefins
ActiveUS20150174565A1Increased formationReduce acid strengthAluminium compoundsCatalytic crackingNaphthaBoiling point
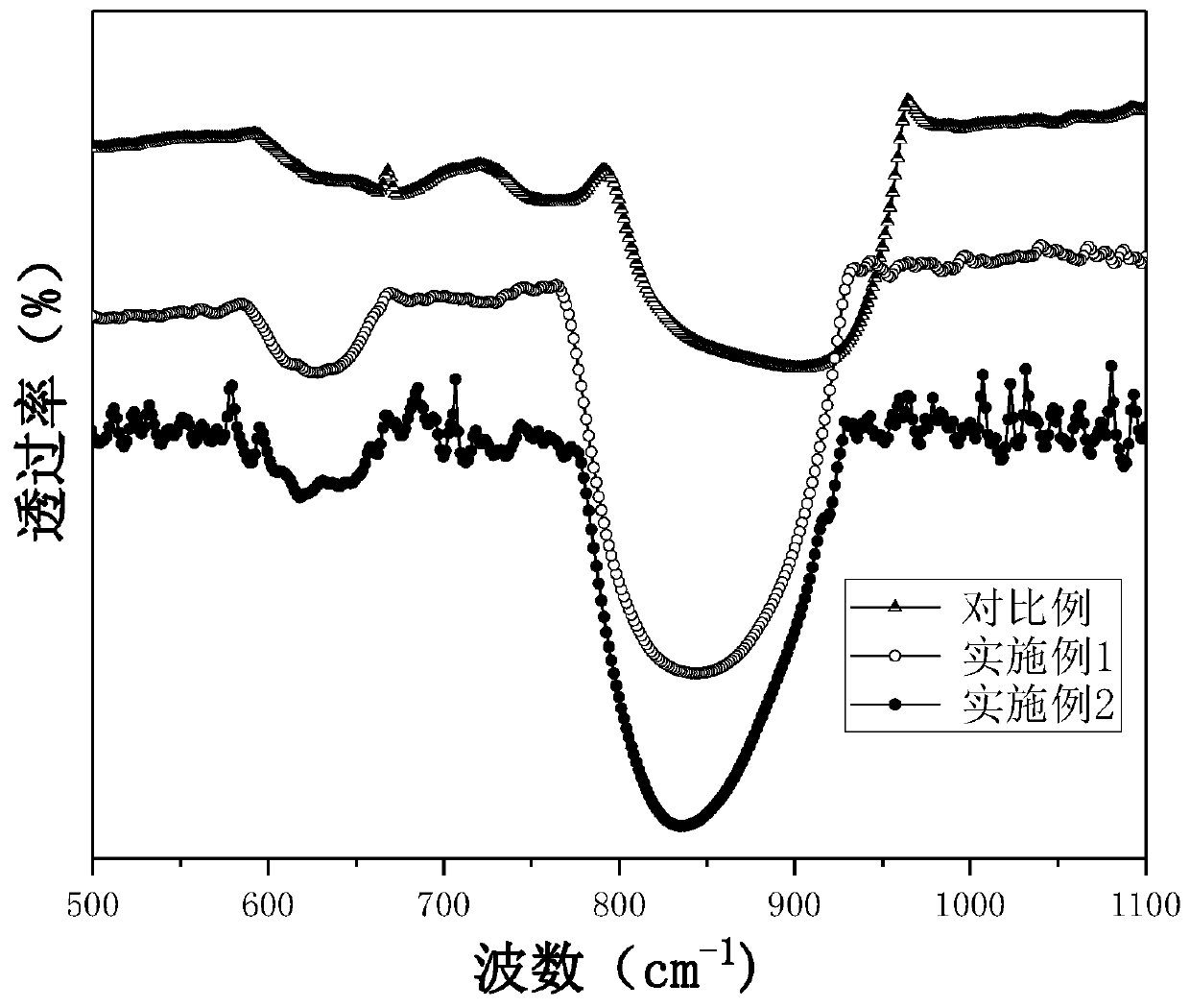
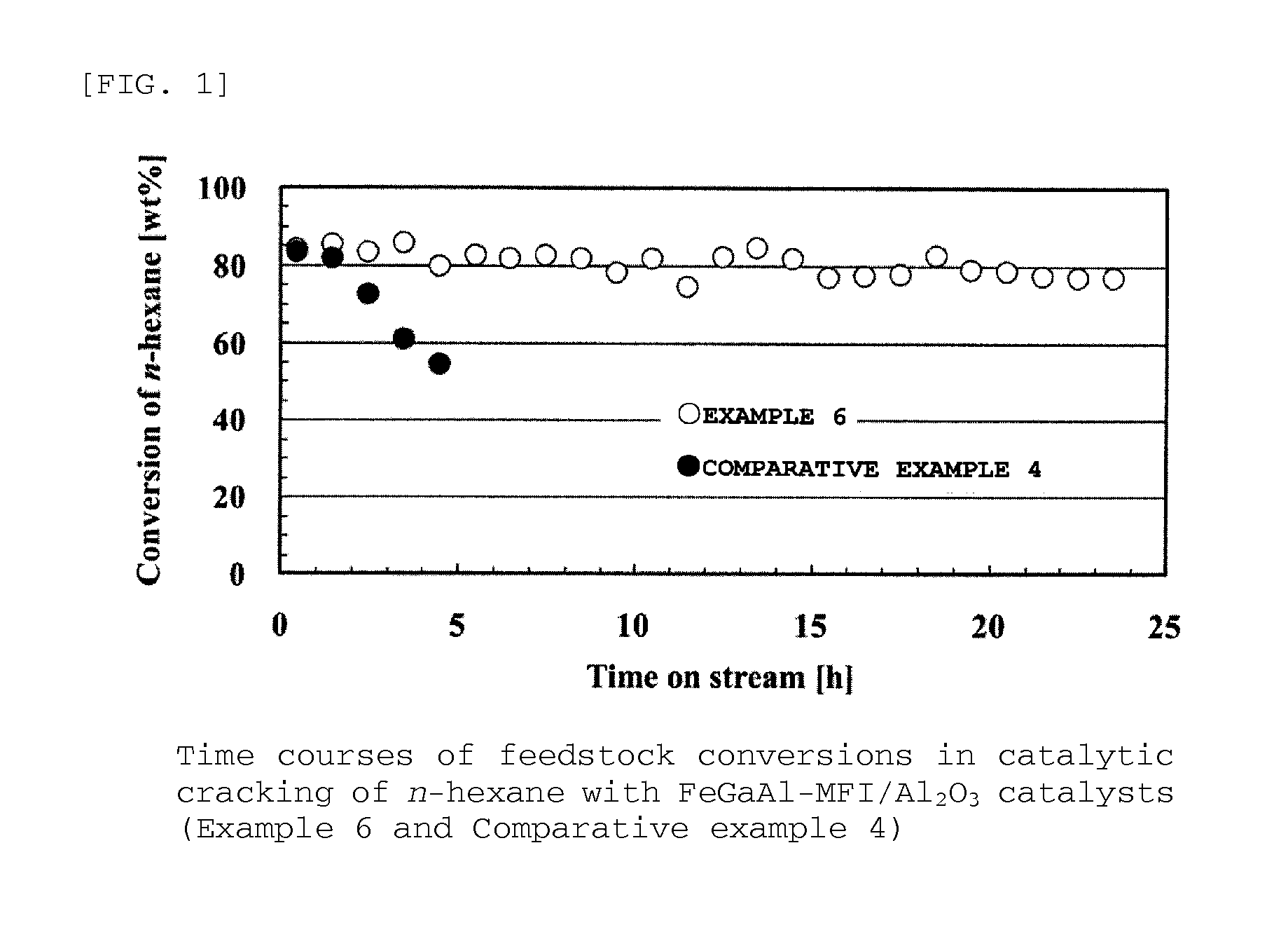
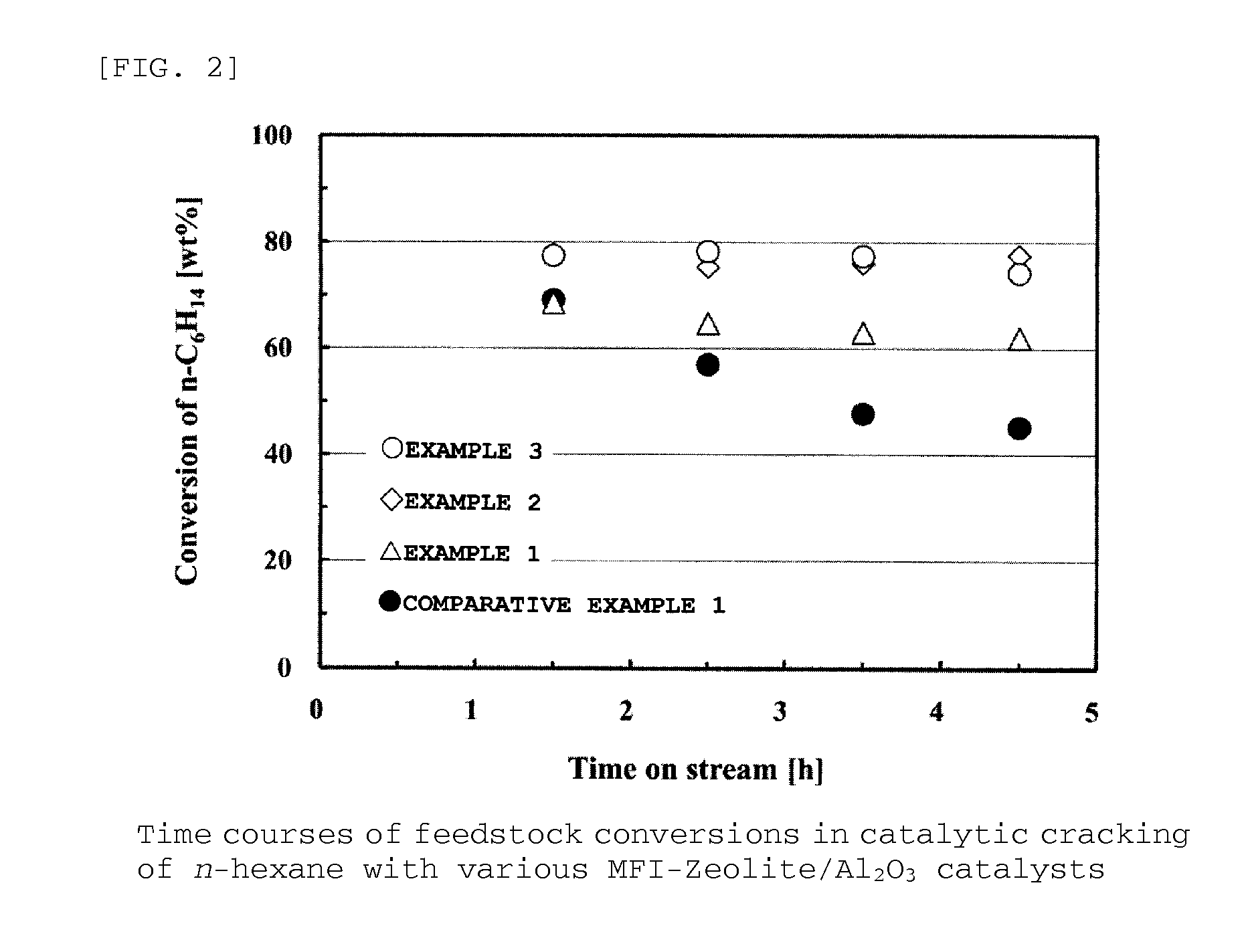
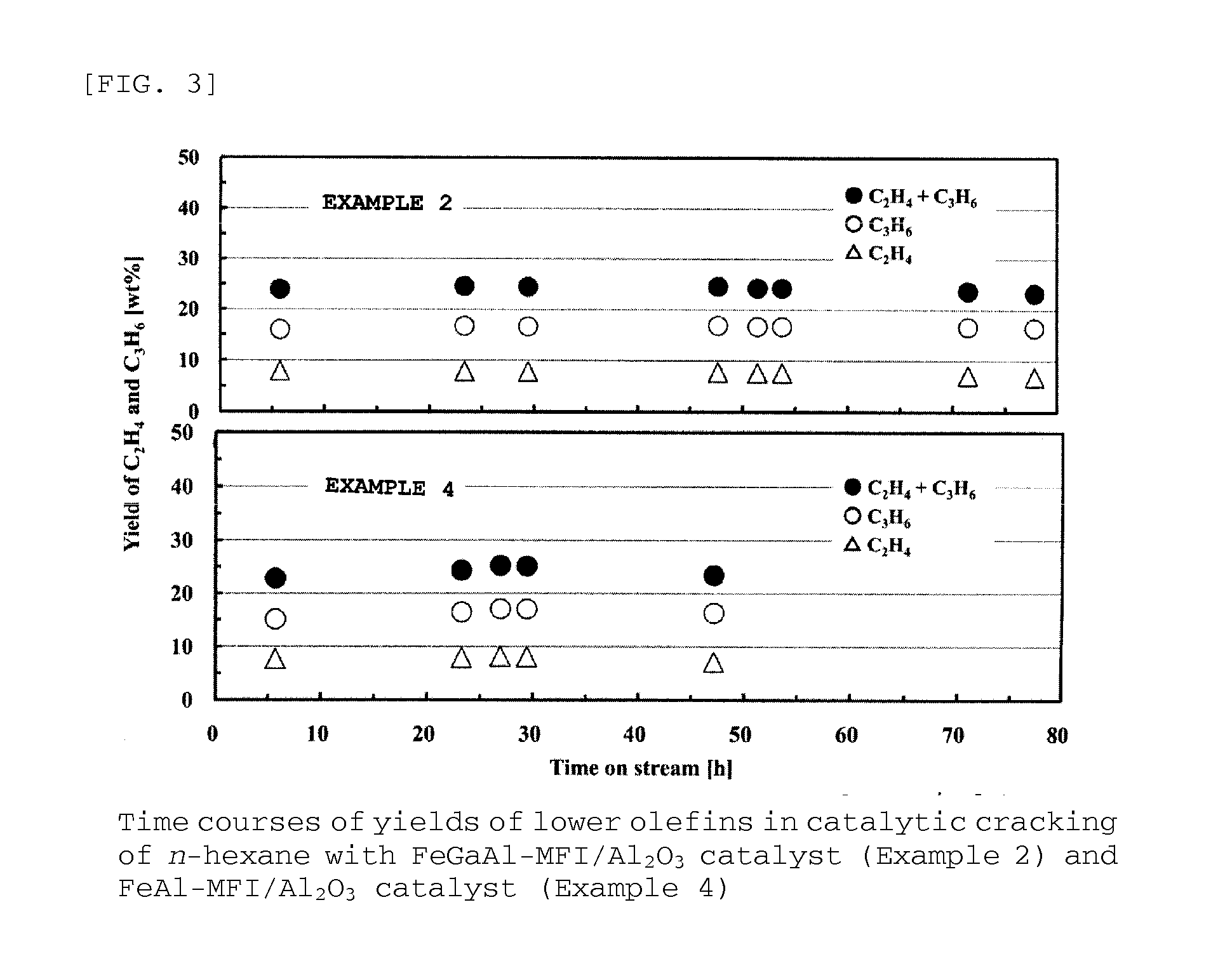
Provided are zeolite catalysts that allow reactions to proceed at temperatures as low as possible when lower olefins are produced from hydrocarbon feedstocks with low boiling points such as light naphtha, make it possible to make propylene yield higher than ethylene yield in the production of lower olefins, and have long lifetime.The zeolite catalysts are used in the production of lower olefins from hydrocarbon feedstocks with low boiling points such as light naphtha. The zeolite catalysts are MFI-type crystalline aluminosilicates containing iron atoms and have molar ratios of iron atoms to total moles of iron atoms and aluminum atoms in the range from 0.4 to 0.7. The use of the zeolite catalysts make it possible to increase propylene yield, to lower reaction temperatures, and to extend catalyst lifetime.
Owner:CHIYODA CORP
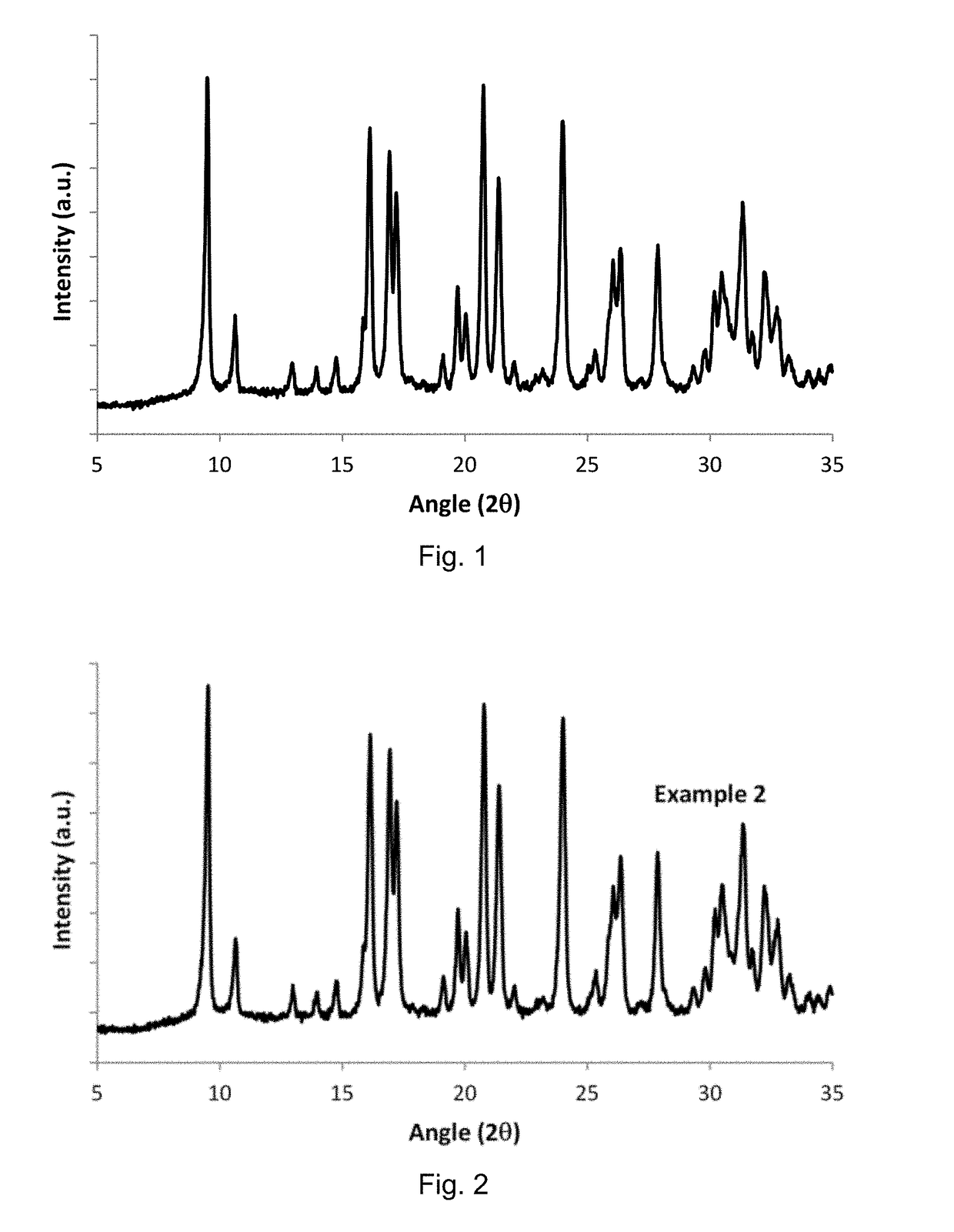
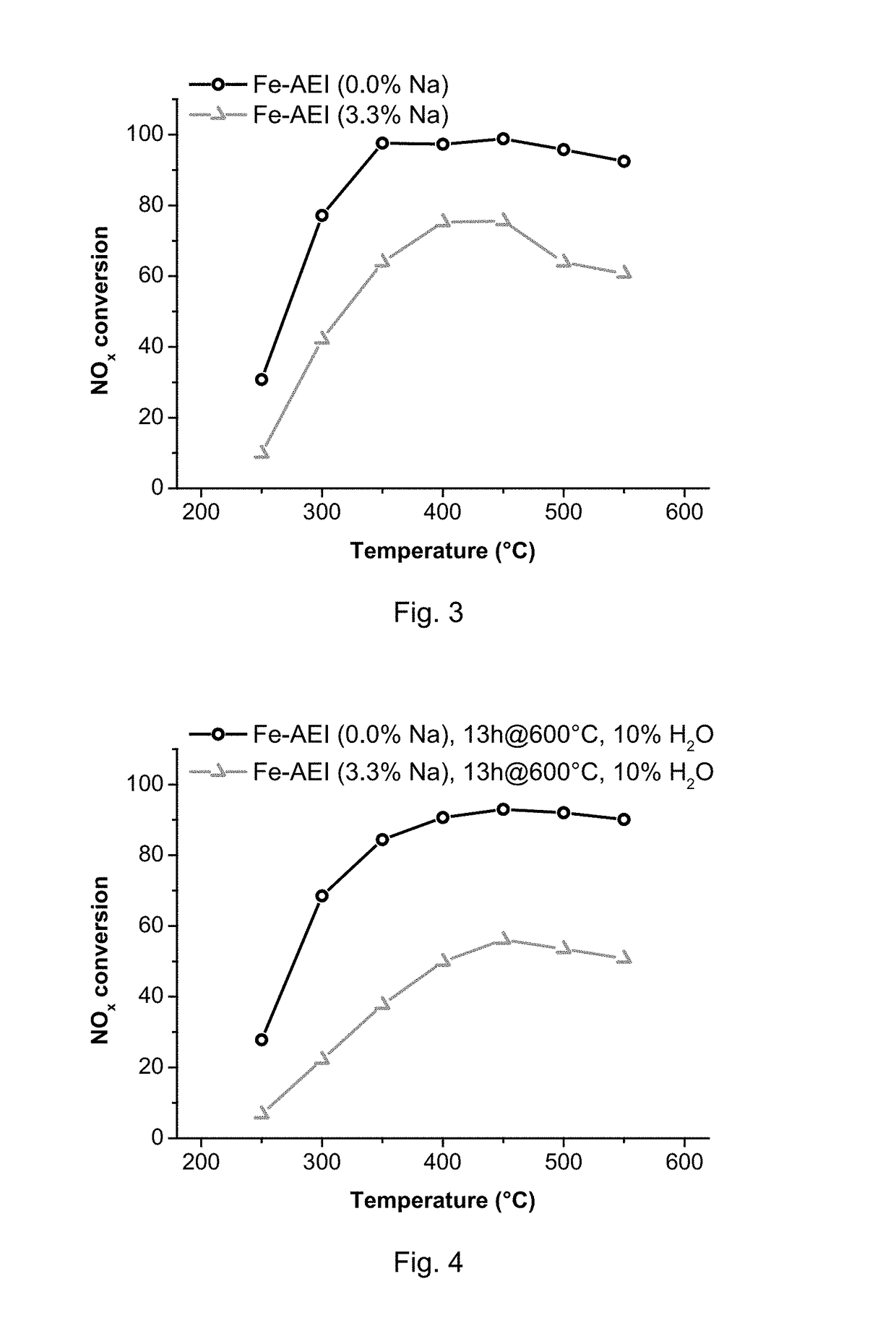
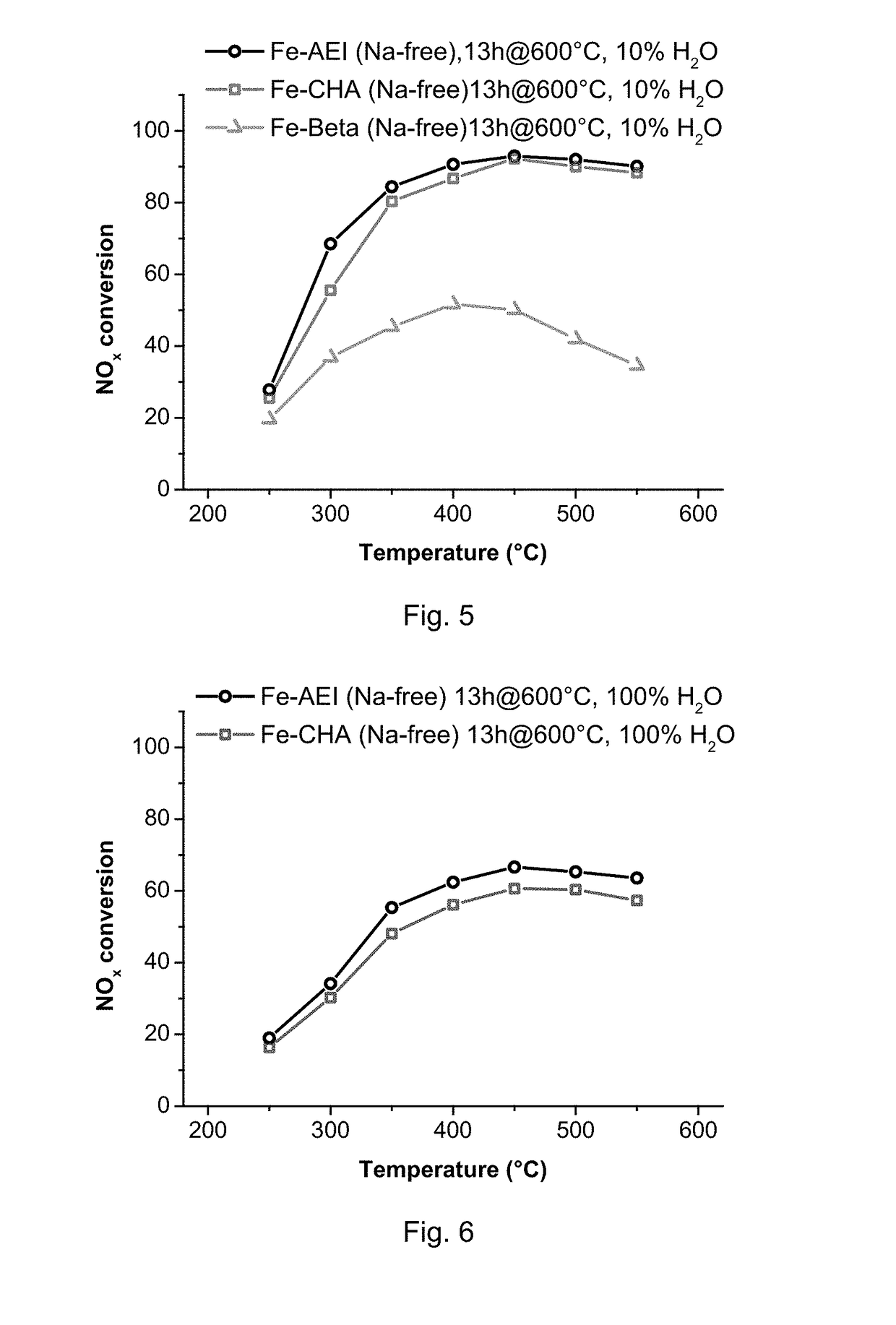
Method for the Direct Synthesis of Iron-Containing AEI-Zeolite Catalyst
InactiveUS20180346341A1Decrease the amount of alkali present in the Fe-AEIMolecular sieve catalystsGalloaluminosilicates/ferroaluminosilicatesAlkali ionsOrganic structure
A method for the direct synthesis of a crystalline material with the AEI zeolithic structure containing iron-species and being essentially free of alkali ions, comprising the following steps:(i) preparation of a mixture containing water, a high-silica zeolite as a main source of silica and alumina, an alkyl-substituted cyclic ammonium cation as organic structure directing agent (OSDA), a source of iron, and a source of an alkali metal ion [Alk], to obtain a final synthesis mixture having the following molar composition:SiO2:aAl2O3:bFe:cOSDA:dAlk:eH2Owherein a is in the range from 0.001 to 0.2;wherein b is in the range from 0.001 to 0.2;wherein c is in the range from 0.01 to 2;wherein d is in the range from 0.001 to 2;wherein e is in the range from 1 to 200;(ii) crystallization of the mixture achieved in (i);(iii) recovery of the crystalline material achieved in (ii);(iv) calcination of the crystalline material from step (iii); and(v) removal of the alkali metal cation, present in the calcined crystalline material after step (iv) to obtain a final molar composition:SiO2:oAl2O3:pFe:qAlkwherein o is in the range from 0.001 to 0.2, p is in the range from 0.001 to 0.2 and q is below 0.02.
Owner:UMICORE AG & CO KG
Processes for producing materials having a zeolite-type framework with heteroatoms incorporated therein
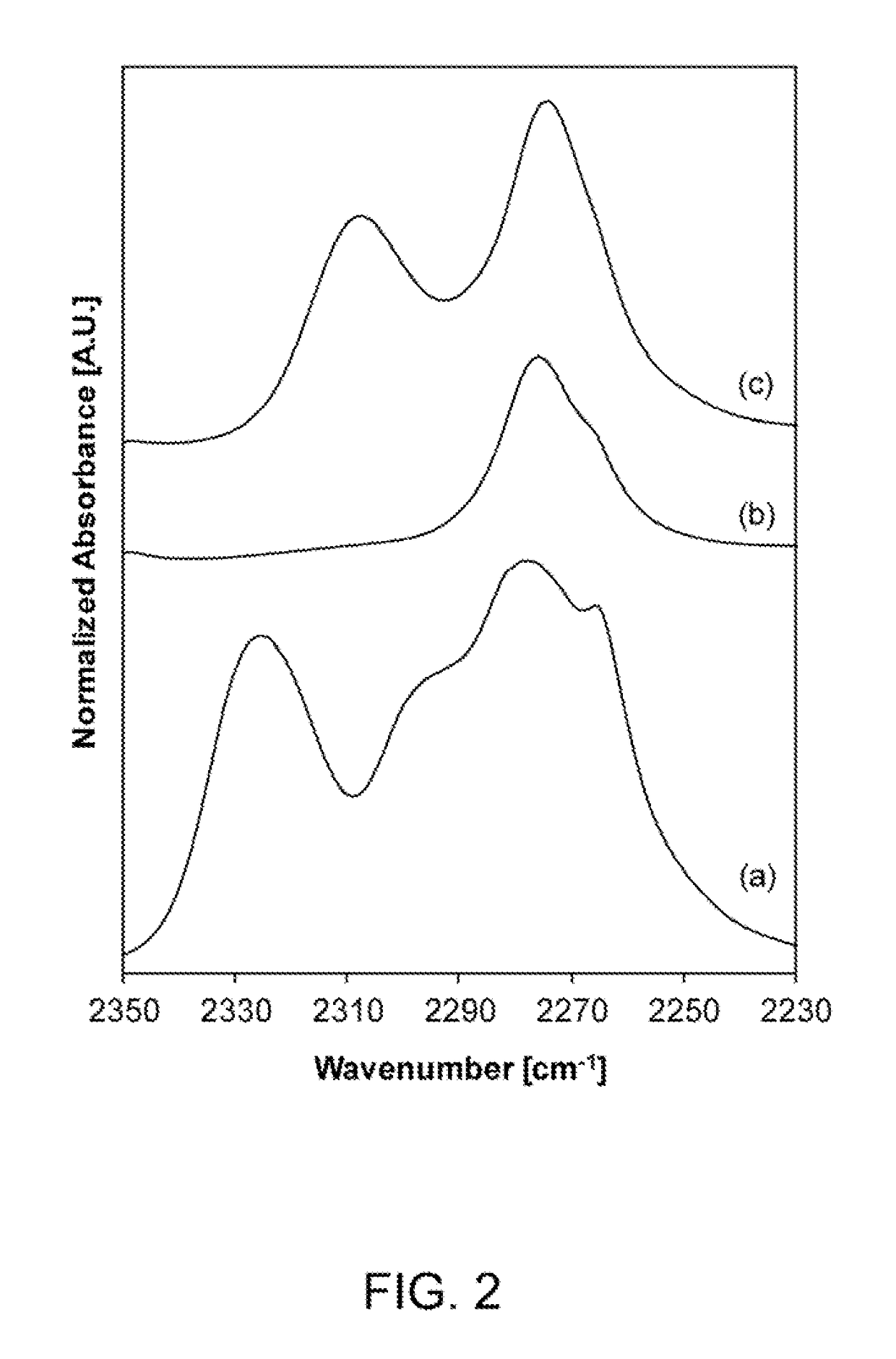
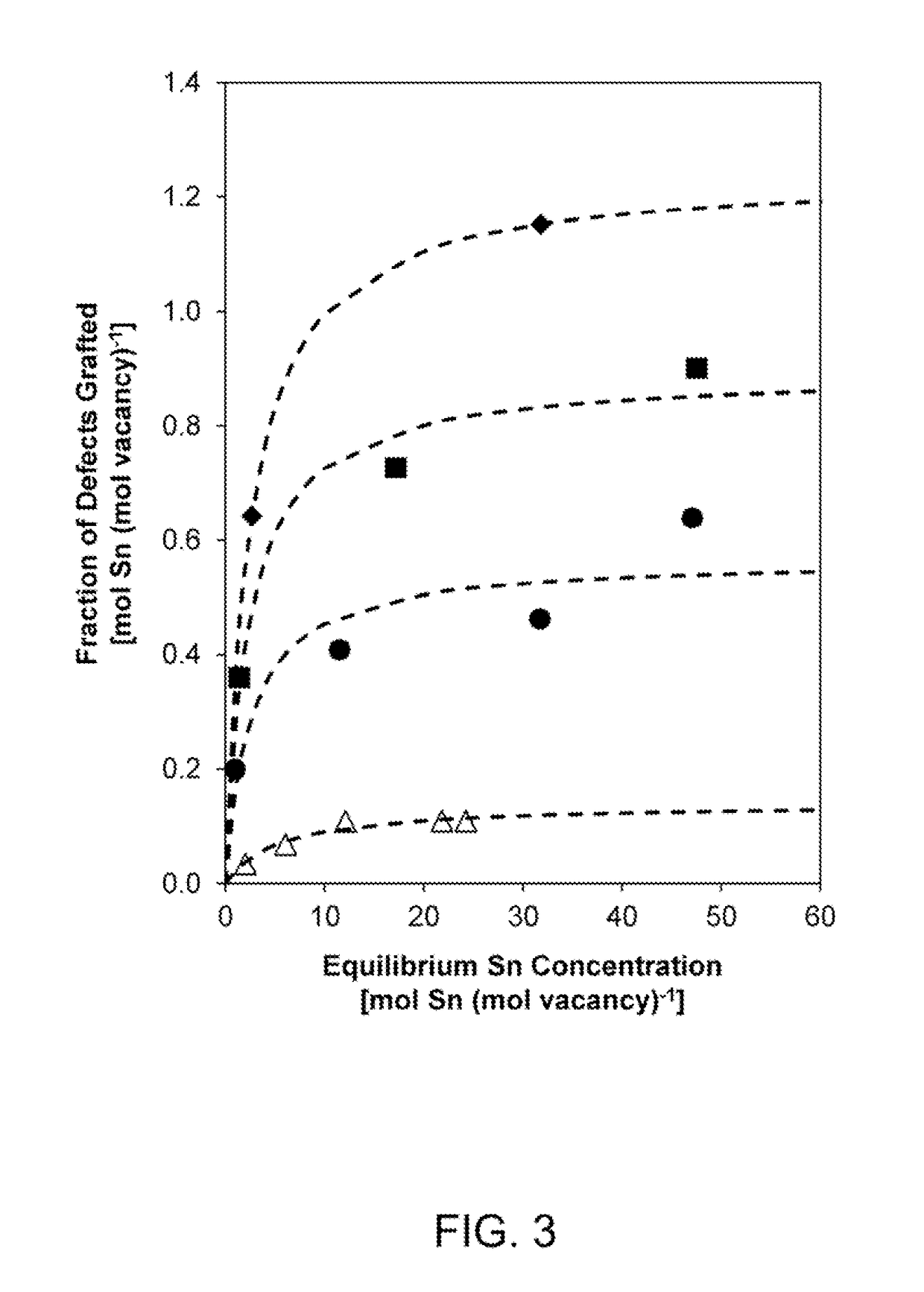
A process of producing a zeotype material having a zeolite-type framework. The process includes providing a zeolite having a framework, dealuminating the zeolite to remove aluminum atoms therefrom to produce a dealuminated framework comprising a plurality of vacancy sites, contacting the dealuminated framework with dichloromethane and a precursor comprising heteroatoms, and then heating the dealuminated framework, the dichloromethane, and the precursor under reflux conditions to incorporate the heteroatoms into at least some of the plurality of vacancy sites in the dealuminated framework to produce a zeotype material having a zeolite-type framework comprising the heteroatoms. In addition, a process is provided for producing a stannosilicate comprising a zeolite-type framework comprising Sn heteroatoms incorporated therein which form Sn sites in the zeolite-type framework each having an open configuration or a closed configuration. This process includes controlling relative amounts of Sn sites having open and closed configurations in the stannosilicate.
Owner:PURDUE RES FOUND INC
Sound absorbing material and speaker box using same
ActiveUS20200037063A1Improve the stability of actionImprove stabilityAluminosilicate zeolite type ZSM-12Molecular-sieve borosilicatesEngineeringAcoustics
The present disclosure provides a sound absorbing material. The sound absorbing material comprises MFI-structural-type zeolite. The MFI-structural-type zeolite comprises a framework, and the framework comprises SiO2 and AlO3, and the mass ratio of Si to Al in the framework is less than 200 and not less than 50. The present disclosure also provides a speaker box applying the sound absorbing material. The sound absorbing material provided by the present disclosure and the speaker box using the sound absorbing material can further improve the performance of the speaker box, reduce the failure of zeolite and improve the performance stability of the speaker box.
Owner:AAC ACOUSTIC TECH (SHENZHEN) CO LTD
Mesoporous silicon-aluminum molecular sieve adsorbent for VOCs (volatile organic chemicals) treatment and preparation method thereof
InactiveCN107055564ALarge specific surface areaLarge hole volumeGas treatmentOther chemical processesSorbentAluminium isopropoxide
The invention discloses a preparation method of a mesoporous silicon-aluminum molecular sieve adsorbent for VOCs (volatile organic chemicals) treatment. Doping modification is performed by taking acetylacetone as a chelating agent, iron nitrate as an iron source and aluminum isopropoxide as an aluminum source; a synthesis route of synthesizing a mesoporous silicon-aluminum molecular sieve by a sol-gel method is adopted. The mesoporous silicon-aluminum molecular sieve provided by the invention is particularly suitable for an adsorbent for VOCs treatment, and has the advantages of large specific surface area, large pore volume, high adsorption selectivity, high mechanical strength, stable chemical properties, wide source and easy regeneration and reutilization.
Owner:JIANGSU WISDOM ENG TECH
Desulfurization adsorbent for fuel cell and desulfurizing method using the same
InactiveUS7842645B2Improve performancePromote regenerationMethane captureMolecular-sieve silicatesAlkaline earth metalSorbent
A desulfurization adsorbent for a fuel cell has a structure according to Formula 1 below, and a desulfurizing method uses the desulfurization adsorbent. The desulfurization adsorbent displays remarkably excellent adsorption performance for adsorbing sulfur compounds as well as excellent regeneration performance, compared with conventional desulfurization adsorbents. Thus, the desulfurization adsorbent does not need to be replaced even after prolonged use, thus stabilizing the operation of a fuel cell system and reducing costs.(M1)a-(Si)x—(Ti)y-(M2)z-O [Formula 1]wherein M1 is at least one selected from alkali metals, alkaline earth metals, hydrogen, ammonium, rare earths, and transition metals; 4≦x / y≦500, 0≦z / y≦3, 0≦a / (y+z)≦1; and M2 is aluminum (Al), boron (B) or a trivalent metal. The desulfurization adsorbent is produced by subjecting a mixture of a silicon source, a titanium source, and optionally, aluminum, boron or a trivalent metal in an alkali solution to a hydrothermal treatment to obtain a crystalline porous molecular sieve.
Owner:SAMSUNG SDI CO LTD
Sound absorbing material, preparation method thereof and loudspeaker using sound absorbing material
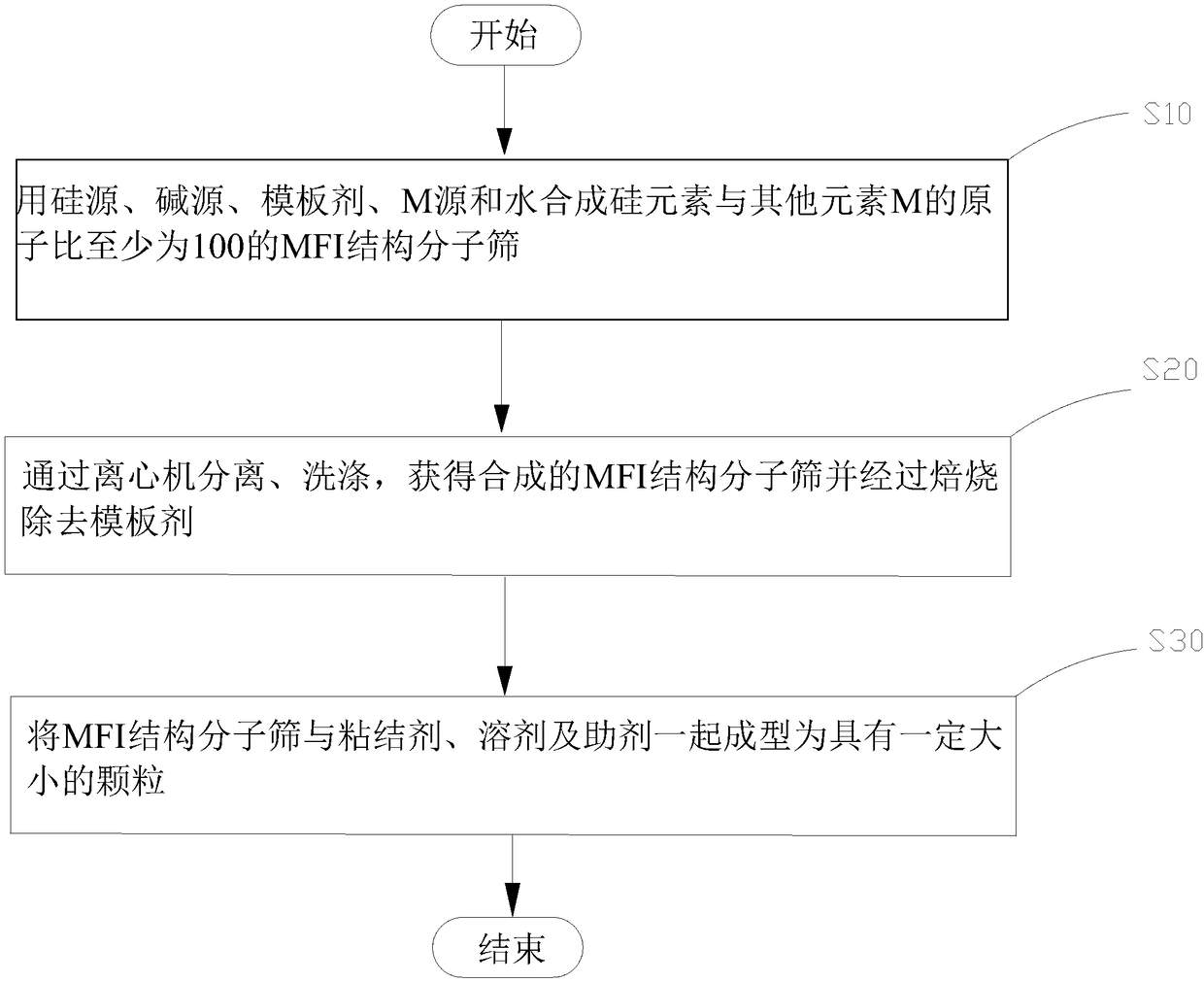
InactiveCN108566593AImprove low frequency effectImprove stabilityElectrical transducersLoudspeakersMolecular sieveAlkaline earth metal
The embodiment of the invention relates to the technical field of sound absorbing materials and discloses a sound absorbing material. In the invention, the sound absorbing material comprises an MFI structure molecular sieve; the MFI structure molecular sieve comprises a framework and framework external positive ions; the framework comprises SiO2 and metal oxide MxOy; the Si / M atomic ratio in the framework is at least 100, wherein M comprises gallium; the framework external positive ions are at least one of hydrogen ions, alkaline metal ions or alkaline-earth metals. The invention also providesa preparation method of the sound absorbing material and a loudspeaker applying the sound absorbing material. According to the sound absorbing material, the preparation method and the loudspeaker applying the sound absorbing material, the performance of the loudspeaker can be improved, the molecular scene invalidation is reduced, and the performance stability of the loudspeaker is improved.
Owner:AAC TECH NANJING
Sound absorbing material and speaker box using same
ActiveUS11109149B2Improve the stability of actionImprove stabilityAluminosilicate zeolite type ZSM-12Molecular-sieve borosilicatesEngineeringMechanical engineering
Owner:AAC ACOUSTIC TECH (SHENZHEN) CO LTD
Aluminosilicate compositions, preparation and use
InactiveUS20060002849A1Useful propertyAdvantageously employedAluminium compoundsAluminium silicatesHigh concentrationMetal
A metalloaluminosilicate composition includes an aluminosilicate composition having an aluminosilicate framework and containing at least one metal, wherein a substantial portion of the metal is incorporated into the aluminosilicate framework. A higher concentration of the metal is incorporated into the framework of the catalyst than is present at the surface of the catalyst.
Owner:INTREVEP SA
Damping material and preparation method thereof as well as loudspeaker enclosure to which damping material is applied
InactiveCN108975347ANot easy to absorb waterHigh room temperature adsorption and desorption air volumeGalloaluminosilicates/ferroaluminosilicatesSound producing devicesMolecular sieveAlkaline earth metal
The embodiment of the invention relates to the technical field of damping materials, and discloses a damping material. The damping material comprises a molecular sieve with an MFI structure, the molecular sieve with the MFI structure comprises a framework and cations outside the framework, the framework comprises SiO2 and metal oxide MxOy which contains a metallic element M, the damping material is characterized in that in the framework, the Si / M atomic mole ratio ranges from 220 to 600, wherein the metallic element M contains aluminum, the cations outside the framework are one or more kind ofhydrogen ions, alkali metal ions or alkaline earth metal. The invention further provides a preparation method of the damping material as well as a loudspeaker enclosure to which the damping materialis applied. According to the damping material and the preparation method thereof as well as the loudspeaker enclosure to which the damping material is applied, the loudspeaker enclosure performance can be further improved, the molecular sieve failure can be reduced, and the performance stability of the loudspeaker enclosure can be improved.
Owner:AAC TECH NANJING
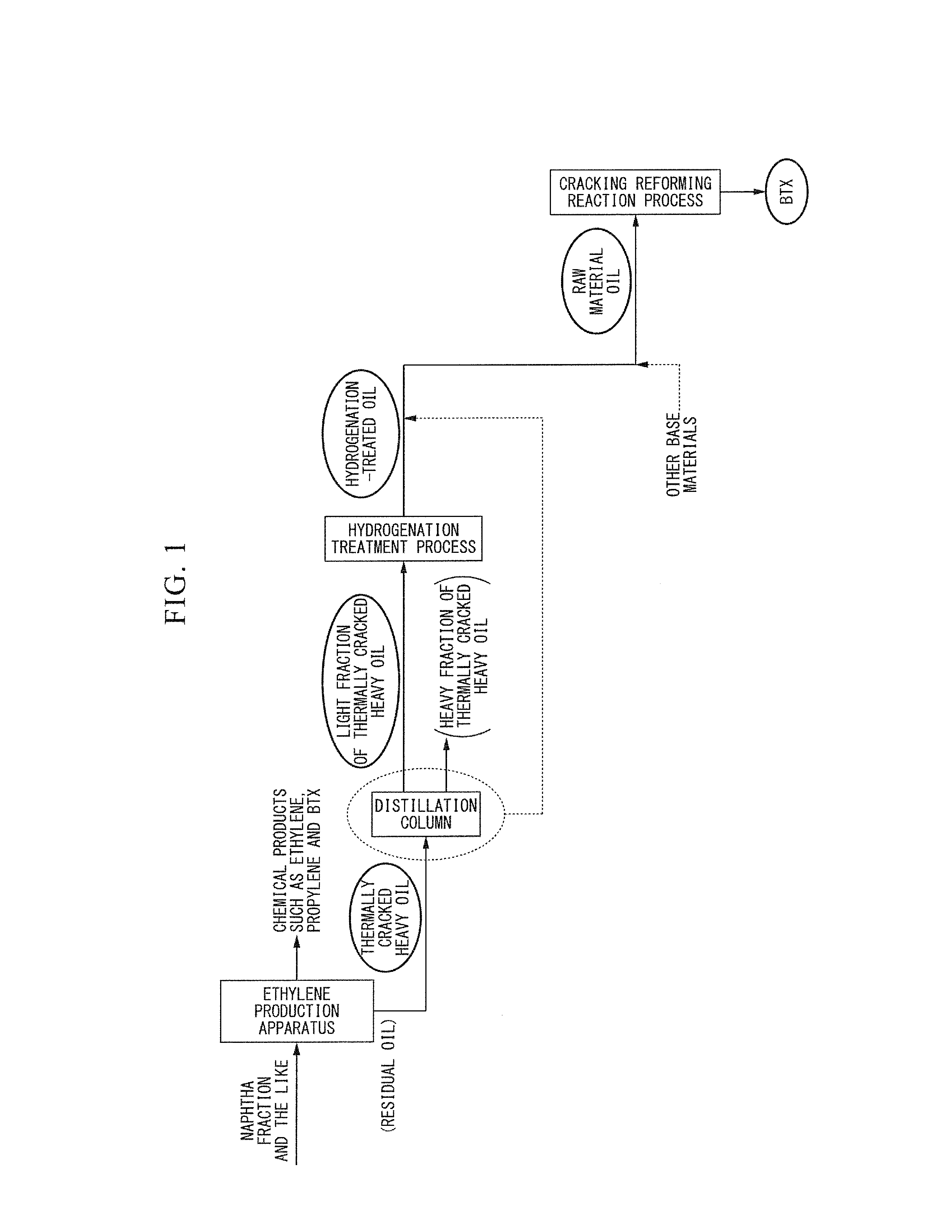
Aromatic hydrocarbon production process
ActiveCN103097496AEfficient preparationThermal non-catalytic crackingOrganic chemistry methodsDistillationAromatic hydrocarbon
This aromatic hydrocarbon production process comprises bringing a raw material oil which contains a hydrogenated oil product of a thermally cracked heavy oil produced in an ethylene production apparatus into contact with a monocyclic aromatic hydrocarbon production catalyst which contains a crystalline aluminosilicate to produce an aromatic hydrocarbon. The raw material oil to be used has a distillation end point temperature of 400 DEG C or lower. The contact between the raw material oil and the monocyclic aromatic hydrocarbon production catalyst is carried out under a pressure of 0.1 to 1.5 MPaG.
Owner:JX NIPPON OIL & ENERGY CORP
Popular searches
Ferrosilicates Sugar derivatives preparation Faujasite aluminosilicate zeolite Silicon compounds Mordenite aluminosilicate zeolite Internal combustion piston engines Molecular sieve catalyst Dispersed particle separation Preparation by carbon monoxide or formate reaction Adsorption purification/separation
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com