Preparation method and application of cholesterol-carboxymethyl chitosan derivative meterials
A technology of carboxymethyl chitosan and cholesterol, which is applied in the field of preparation of cholesterol-carboxymethyl chitosan derivative materials, can solve the problems of no induced release function, low compatibility, non-natural degradation, etc. Good slow release controllability, reduce environmental pollution, and expand the effect of compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
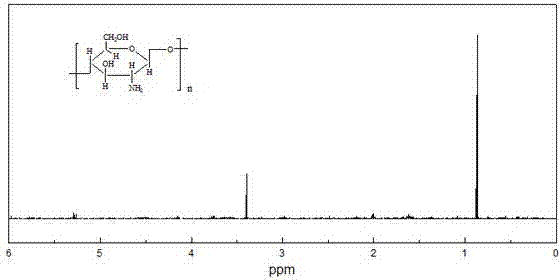
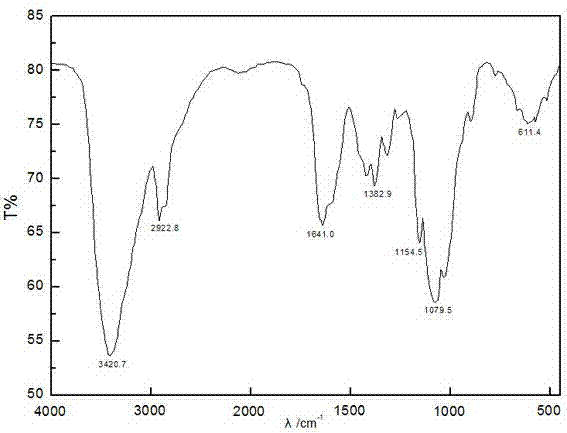
[0041] (1) Soak 1g of chitosan in isopropanol, add 50wt% NaOH solution in 3 times, and finish adding in 10 minutes, stir at 55°C for 50min to form a pH=12 alkaline environment, and then divide it into 3 times Add 6g of chloroacetic acid once, and add it within 10, and chitosan C 6 For the -OH reaction on the surface, keep the temperature change within the range of 5°C, react for 2.5h, and reflux the ethanol with a volume concentration of 95%, and carry out the hydrazinolysis reaction with a hydrazine hydrate solution with a mass percentage concentration of 85wt%, so that the phthal The imido group is deprotected to form O-carboxymethyl chitosan;
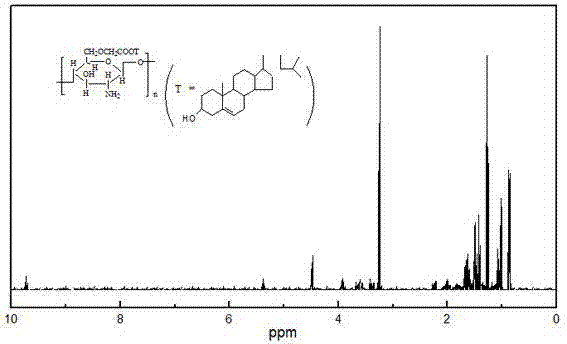
[0042](2) O-carboxymethyl chitosan 0.6g is dissolved in the acetic acid solution that volume ratio is 2%, 0.6g cholesterol is dissolved in the dimethyl formamide, activator carbodiimide 0.3g joins in In the dimethylformamide solution, stir for 25min and slowly drop the mixed solution into the O-carboxymethyl chitosan acetic acid sol...
Embodiment 2
[0045] (1) Soak 1g of chitosan in isopropanol, add 50wt% NaOH solution in 5 times, and finish adding in 15 minutes, stir at 60°C for 55min to form a pH=13 alkaline environment, and then divide into 3 Add 4.5g of chloroacetic acid once, and add it within 15, and chitosan C 6 For the -OH reaction on the surface, keep the temperature change within 5°C, react for 3h, reflux through ethanol with a volume concentration of 95%, and use 85wt% hydrazine hydrate solution for hydrazinolysis reaction to remove the phthalimide group Protection, forming O-carboxymethyl chitosan;
[0046] (2) Dissolving 0.6g of O-carboxymethyl chitosan in 3% acetic acid solution by volume, dissolving 0.6g of cholesterol in dimethylformamide, and dissolving activator N-hydroxysuccinimide 0.24 g was added to the dimethylformamide solution, stirred for 30 minutes and slowly added dropwise the mixed solution to the O-carboxymethyl chitosan solution under magnetic stirring, reacted at room temperature for 2.5 da...
Embodiment 3
[0048] (1) Soak 1g of chitosan in isopropanol, add 50wt% NaOH solution in 4 times, and finish adding in 20 minutes, stir at 65°C for 50min to form a pH=14 alkaline environment, and then divide into 3 Add 12g of chloroacetic acid once, and add it within 20, and chitosan C 6 For the -OH reaction on the surface, keep the temperature change within 5°C, react for 3h, reflux through ethanol with a volume concentration of 95%, and use 85wt% hydrazine hydrate solution for hydrazinolysis reaction to remove the phthalimide group Protection, forming O-carboxymethyl chitosan;
[0049] (2) O-carboxymethyl chitosan 0.6g is dissolved in the acetic acid solution that volume ratio is 4%, and 0.6g cholesterol is dissolved in the dimethylformamide, and activator carbodiimide 0.1g, N Under the action of 0.1g of hydroxysuccinimide, add it to the dimethylformamide solution, stir for 35min and slowly add the mixed solution dropwise to the O-carboxymethyl chitosan acetate solution under magnetic sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com