A gene-knockout Escherichia coli beneficial to extracellular secretion of recombinant protein and its application
A technology of Escherichia coli and gene knockout, which is applied in application, genetic engineering, plant gene improvement, etc., can solve the problems of low extracellular expression and increase downstream costs, and achieve enhanced extracellular secretion, low cost, and improved yield and purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] This embodiment illustrates the construction process of Escherichia coli that knocks out the waaF gene, which specifically includes the following steps:
[0039] (1) The gene waaF (SEQ ID NO: 1) of lipopolysaccharide heptose transferase II in Escherichia coli BL21 (DE3) was first cloned by PCR using waaF gene cloning primers waaF-F and waaF-R, and connected to the pMD18T vector A recombinant plasmid was constructed on the above and named pMD18T-waaF;
[0040] (2) Using the pMD18T-waaF recombinant plasmid obtained in step (1) as a template, perform reverse PCR with reverse primers fxwaaF-F and fxwaaF-R, remove the sequence in the middle of waaF, and amplify the two ends of the waaF gene with a length of about 220bp The homologous sequence of the inverse PCR product and the resistance fragment dif-Gm r -dif link, i.e. in the resistant fragment dif-Gm r The two ends of -dif are respectively connected with the homologous sequence of the waaF gene to obtain the fusion gene...
Embodiment 2
[0046] This example illustrates the method for constructing fructosidase-producing bacteria using Escherichia coli knockout of the waaF gene.
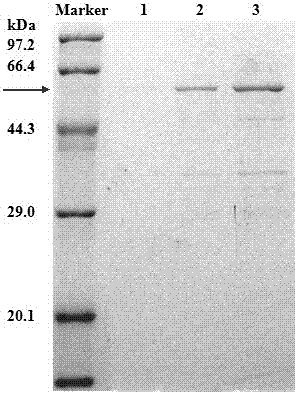
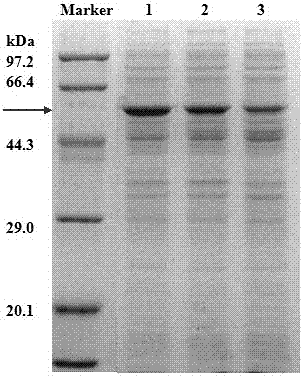
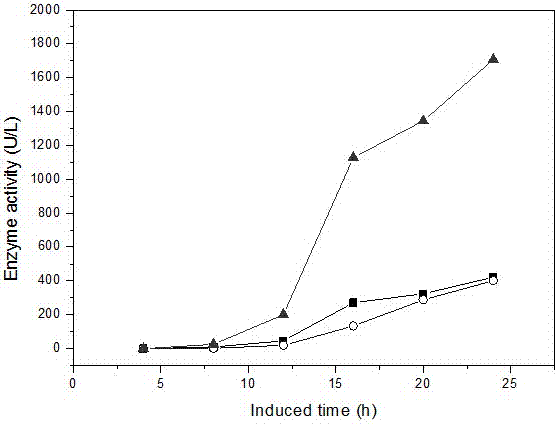
[0047] The gene fragment Fru of fructosidase with its own signal peptide (disclosed in the invention patent of patent No. ZL201210185733.8, named an organic solvent-resistant glycosidase Fru6 and its gene and application, the sequence is as SEQ in this patent ID NO: shown in 1) was cloned into the plasmid pET22b (+) to obtain the recombinant plasmid pET22b (+)-Fru, and then transformed into the Escherichia coli strain (prepared in Example 1) that knocked out the waaF gene to obtain Escherichia coli BL21 ( DE3)::ΔwaaF / pET22b(+)-Fru.
Embodiment 3
[0049] This example illustrates the method for constructing a penicillin G acylase-producing bacterium using Escherichia coli in which the waaF gene has been knocked out.
[0050] The gene fragment pga (Cheng TF et al. Protein Expression and Purification, 2006,46 (1): 107-113.) of the penicillin G acylase derived from Escherichia coli was cloned in the plasmid pET22b (+), and the recombinant plasmid pET22b ( +)-pga, and then transformed into the Escherichia coli strain (prepared in Example 1) where the waaF gene was knocked out to obtain Escherichia coli BL21(DE3)::ΔwaaF / pET22b(+)-pga.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com