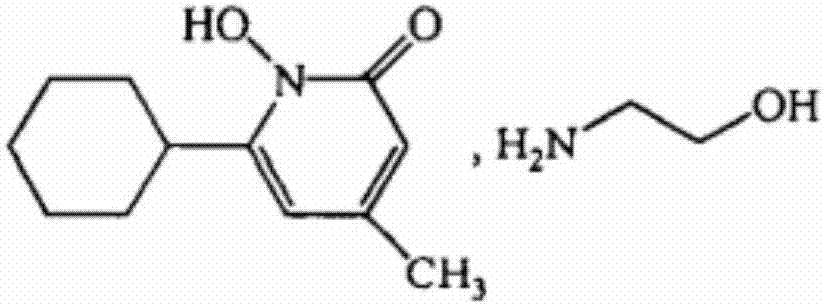
Ciclopirox olamine vaginal suppository and its preparation method
A kind of technology of ciclopirox amine and vaginal suppository, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: Preparation of ciclopirox olamine suppository
[0086] formula:
[0087] Ciclopirox olamine 100mg,
[0088] Mixed fatty acid glycerides 2000mg.
[0089] Preparation method:
[0090] (1) Pre-crushing and sieving the raw and auxiliary materials for subsequent use;
[0091] (2) Mix ciclopirox olamine with part (50% of the formulation amount) of the matrix evenly, and heat to 80-85°C under stirring until the material is completely melted, and the active ingredient is evenly dispersed in the matrix;
[0092] (3) The rest of the matrix powder is placed in the batching tank, and under rapid stirring of the matrix powder, slowly pour the molten material obtained in step (2) into the stirred matrix powder. After mixing, continue stirring Raise the temperature of all materials to 55-60°C and maintain at this temperature for 5 hours;
[0093] (4) Then, under stirring, lower the temperature of the material to 45-50°C and maintain this temperature, pour the mate...
Embodiment 2
[0095] Embodiment 2: Preparation of ciclopirox amine suppository
[0096] formula:
[0097] Ciclopirox olamine 100mg,
[0098] Mixed fatty acid glycerides 1000mg.
[0099] Preparation method:
[0100] (1) Pre-crushing and sieving the raw and auxiliary materials for subsequent use;
[0101] (2) Mix the ciclopirox olamine with part (60% of the formulation amount) of the matrix evenly, and heat to 80-85°C under stirring until the material is completely melted, and the active ingredient is evenly dispersed in the matrix;
[0102] (3) The rest of the matrix powder is placed in the batching tank, and under rapid stirring of the matrix powder, slowly pour the molten material obtained in step (2) into the stirred matrix powder. After mixing, continue stirring Raise the temperature of all materials to 55-60°C and maintain this temperature for 4 hours;
[0103] (4) Then, under stirring, lower the temperature of the material to 45-50°C and maintain this temperature, pour the mater...
Embodiment 3
[0105] Embodiment 3: preparation ciclopirox olamine suppository
[0106] formula:
[0107] Ciclopirox olamine 100mg,
[0108] Mixed fatty acid glycerides 5000mg.
[0109] Preparation method:
[0110] (1) Pre-crushing and sieving the raw and auxiliary materials for subsequent use;
[0111] (2) Mix ciclopirox olamine with part (40% of the formulation amount) of the matrix evenly, and heat to 80-85°C under stirring until the material is completely melted, and the active ingredient is evenly dispersed in the matrix;
[0112] (3) The rest of the matrix powder is placed in the batching tank, and under rapid stirring of the matrix powder, slowly pour the molten material obtained in step (2) into the stirred matrix powder. After mixing, continue stirring Raise the temperature of all materials to 55-60°C and maintain at this temperature for 6 hours;
[0113] (4) Then, under stirring, lower the temperature of the material to 45-50°C and maintain this temperature, pour the materia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com