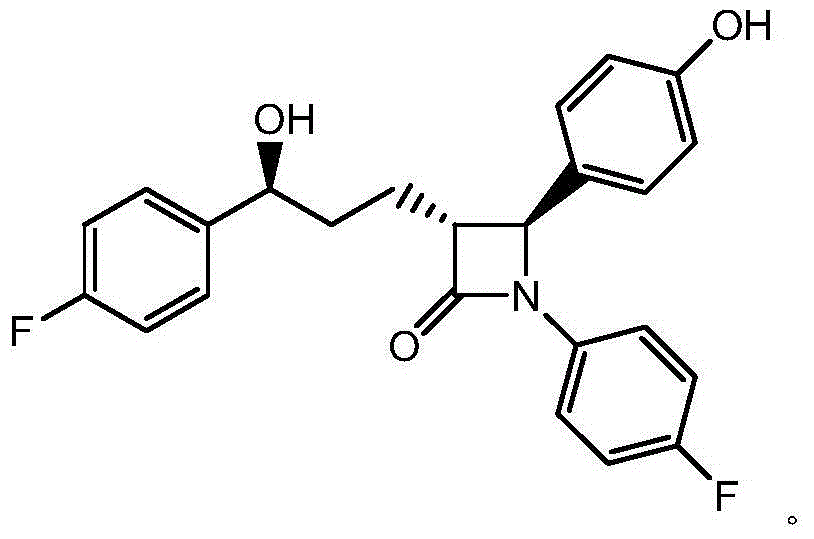
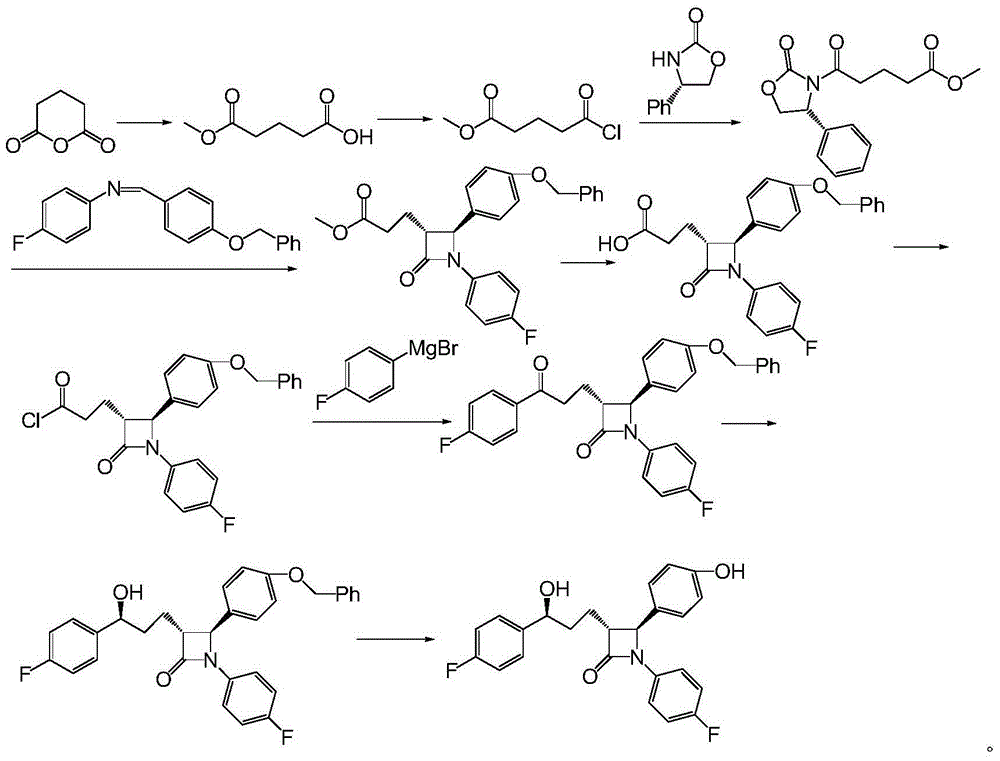
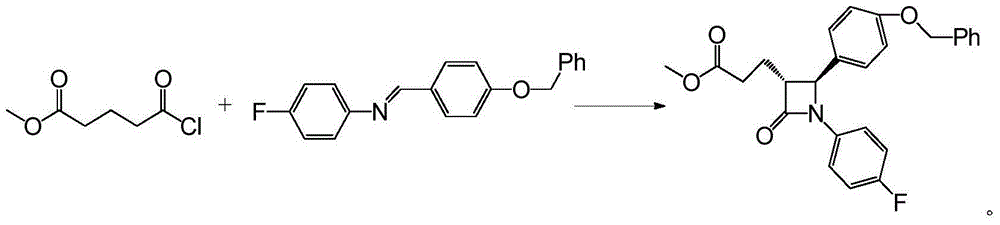
Ezetimibe synthesis intermediate and preparation method and application thereof
A technology of ezetimibe and intermediates, applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, organic chemistry, etc., can solve the difference in the sequence of chiral reduction, low industrial application prospects, The problems of complicated operation in the process can achieve the effect of simple operation, good industrial application prospect and high optical purity of the product.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] With stirring, 20 g of the substrate Add to the reaction solution containing the following components: 700mL water, 2g of aldehyde and ketone reductase lyophilized powder, 7g of glucose and 500U of glucose dehydrogenase (purchased from sigma), and 0.1mM NADP+, and stirred at 30°C for 16 hours During the period, the pH value of the system was controlled between 6.5-7.5 by using 1M NaOH aqueous solution, and the reaction progress was detected by TLC.
[0064] After the reaction, adjust the pH value to about 9.0, extract 3 times with an equal volume of ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, spin down the solvent under reduced pressure to obtain 18.5 g of the product, the yield is 91.8%, and the purity is 97.0 %.
[0065] The structure of the product was confirmed by 1H NMR, electrospray ionization mass spectrometry and e.e. value determination.
[0066]
[0067] 1 H NMR (300MHz, CDCl3): δ6.66-7.02 (4H, m, Ar-H), 5.14 (1H, t, -C...
Embodiment 2-4
[0071] Referring to the method of Example 1, using the substrates listed in Table 1, Examples 2-4 were carried out.
[0072] The results are shown in Table 1.
[0073] Table 1
[0074]
Embodiment 5
[0076] With stirring, 20 g of the substrate Add to the reaction solution containing the following components: 700mL water, 200mL crude enzyme solution and 20mL isopropanol, stir and react at 30°C for 16 hours, during which the pH value of the system is controlled between 6.5-7.5 with 1M NaOH aqueous solution, TLC detects the progress of the reaction.
[0077] After the reaction, adjust the pH value to 9.0, extract 3 times with an equal volume of ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, spin down the solvent under reduced pressure to obtain 18.3 g of the product, the yield is 90.8%, and the purity is 96.9% .
[0078] The structure of the product was confirmed by 1H NMR, electrospray ionization mass spectrometry and e.e. value determination.
[0079]
[0080] 1 H NMR (300MHz, CDCl3): δ6.66-7.02 (4H, m, Ar-H), 5.14 (1H, t, -CHOH), 3.67 (3H, s, -OCH 3 ),2.61-2.86(2H,d,-CH 2 CO 2 CH 3 ),2.0(1H,s,-OH),0.08(9H,s,-Si(CH 3 ) 3 ).
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com