Dual-core ionic iridium coordination compound and uses thereof
A technology of iridium complex and ionic type is applied in the field of binuclear ionic iridium complex to achieve the effect of saving organic solvent, short synthesis route and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
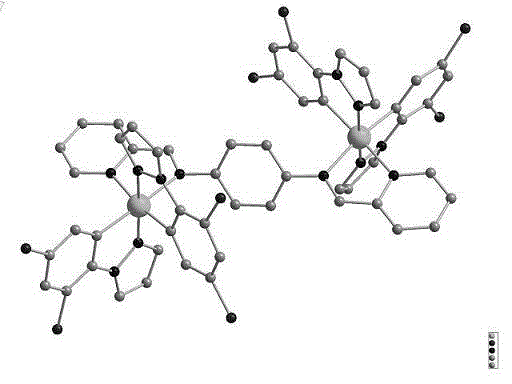
[0031] Mix 0.1043 g (0.1 mmol) of 2-fluorophenylpyrazole dichloride bridge with 0.0284 g (0.1 mmol) of ligand N,N'-(di-2-pyridyl)-1,4-diiminobenzene In a 50ml round-bottom flask, add 15ml of methanol and 15ml of dichloromethane as solvents respectively, and stir and reflux for 4h at 78°C under nitrogen protection. After the reaction, cool to room temperature and add 0.0368g of potassium hexafluorophosphate (0.2 mmol), stirred for 1 hour, and then filtered to obtain 0.1340 g (79.8%) of orange solid powder. The molecular formula is C 54 H 34 F 20 Ir 2 N 12 P 2 , The relative molecular mass is 1678.14g / mol, and the melting point of the complex is 385℃. Single crystal structure diagram such as figure 1 Shown.
Embodiment 2
[0033] Mix 0.1043 g (0.1 mmol) of 2-fluorophenylpyrazole dichloride bridge with 0.0341 g (0.12 mmol) of ligand N,N'-(di-2-pyridyl)-1,4-diiminobenzene In a 50ml round-bottom flask, add 15ml of methanol and 15ml of dichloromethane as solvents respectively, and stir and reflux for 4h at 78°C under nitrogen protection. After the reaction, cool to room temperature and add 0.0368g of potassium hexafluorophosphate (0.2 mmol), stirred for 1 hour, then filtered to obtain 0.1450g (86.4%) of orange solid powder; the molecular formula is C 54 H 34 F 20 Ir 2 N 12 P 2 , The relative molecular mass is 1678.14g / mol, and the melting point of the complex is 385℃. Single crystal structure diagram such as figure 1 Shown.
Embodiment 3
[0035] Mix 0.2086 g (0.2 mmol) of 2-fluorophenylpyrazole dichloride bridge with 0.0625 g (0.22 mmol) of ligand N,N'-(di-2-pyridyl)-1,4-diiminobenzene In a 50ml round-bottom flask, add the same amount of methanol 15ml and dichloromethane 15ml respectively as solvents, stir and reflux for 4h at 78°C in the dark under nitrogen protection. After the reaction, cool to room temperature and add 0.0736g of potassium hexafluorophosphate (0.4 mmol), stirred for 1 hour, and then filtered to obtain 0.2688g (80.1%) of orange solid powder; the molecular formula is C 54 H 34 F 20 Ir 2 N 12 P 2 The relative molecular mass is 1678.14g / mol, and the melting point of the complex is 385℃. Single crystal structure diagram such as figure 1 Shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Sub-mass | aaaaa | aaaaa |
| Molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com