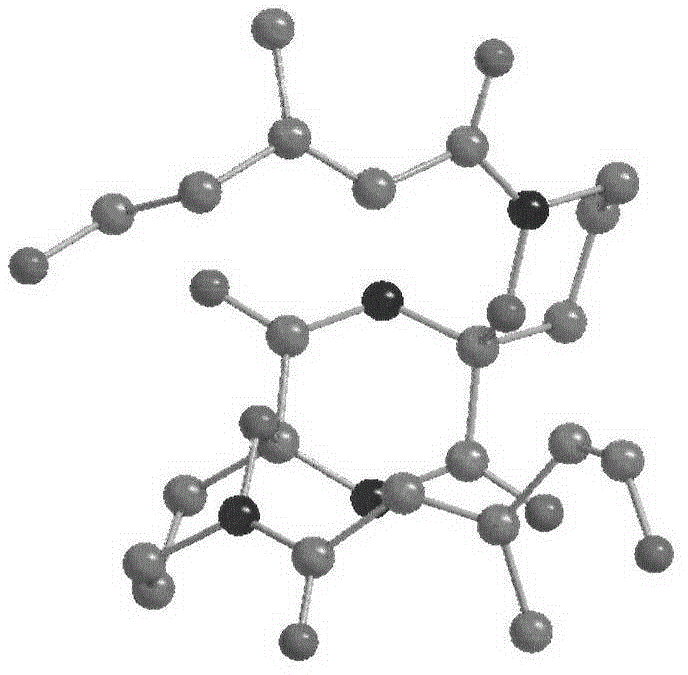
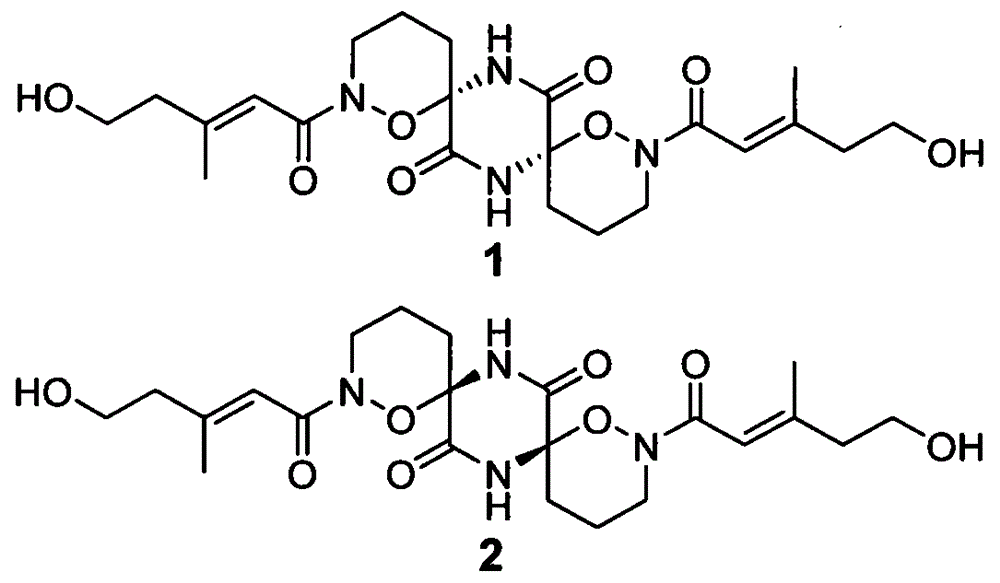
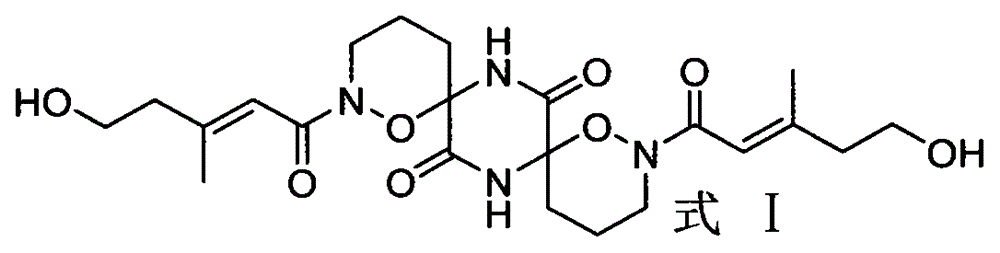
Alkaloid dimer, preparation method thereof and application of alkaloid dimer as antiviral agent
An alkaloid dimer, antiviral agent technology, applied in biochemical equipment and methods, antiviral agents, microorganism-based methods, etc., to achieve the effect of broad application prospects and strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Strain culture of soft coral endophytic fungus Pestalotiopsis sp. (ZJ-2009-7-6)
[0020] The culture medium used for strain culture contains glucose 1.0% (percentage by weight, the same below), yeast extract 0.2%, peptone 0.2%, agar 1.0%, sodium chloride 3.0%, and the rest is water. Strains were cultured at 30°C for 3 days.
[0021] (2) Fermentation of soft coral endophytic fungus Pestalotiopsis sp. (ZJ-2009-7-6)
[0022] The medium used for the fermentation culture contains 40.0% rice (percentage by weight, the same below), 3.0% sodium chloride, 0.2% calcium chloride, 2% potassium bromide, and the rest is water; the fungal strains are cultivated at 28° C. for 90 days.
[0023] (3) extraction and separation of formula I compound
[0024] Get 60 bottles of mycelia obtained in step (2), extract 3 times with chloroform-methanol mixed solution (1:1) and concentrate under reduced pressure, then extract 3 times with ethyl acetate to obtain crude extract; Extraction was...
Embodiment 2
[0030] (1) Strain culture of soft coral endophytic fungus Pestalotiopsis sp. (ZJ-2009-7-6)
[0031] The culture medium used for strain cultivation contains 0.1%-5.0% of glucose (percentage by weight, the same below), 0.01%-1% of yeast extract, 0.01%-1% of peptone, 0.1%-3.0% of agar, and 0.05% of sodium chloride- 5%, and the rest is water. When used, it is made into a test tube slant, and the fungal strains are cultivated at 0-30°C for 3-15 days.
[0032] (2) Fermentation of soft coral endophytic fungus Pestalotiopsis sp. (ZJ-2009-7-6)
[0033] The medium used for fermentation culture contains rice 1.0%-80.0% (weight percentage, the same below), sodium chloride 0.05%-5%, calcium chloride 0.01%-5%, potassium bromide 0.01%-5%, and the rest are In water, fungal strains are cultured at 0-30°C for 20-100 days.
[0034] (3) extraction and separation of formula I compound
[0035] Take 10-60 bottles of the obtained mycelium obtained in step (2), extract the obtained mycelium with c...
Embodiment 3
[0039] Take 1.5 mg of the compound of formula I and dissolve it in a vial filled with 500 μL of methanol, ethanol, water, tetrahydrofuran or acetone. After standing at 0° C. for 30 days, slowly crystallize to obtain the crystal of the compound of formula I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com