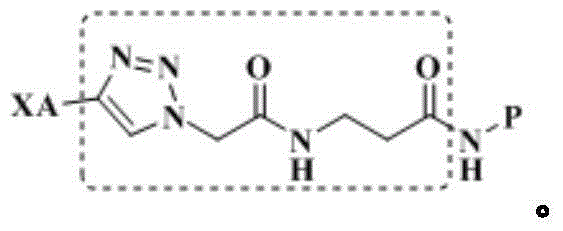
Triterpene-polypeptide conjugate, drug composition and uses thereof
A technology of compounds and medicinal salts, applied in the direction of fusion of polypeptides, peptides, peptide sources, etc., can solve problems such as low resistance, weak activity, and easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] Embodiment 1: the preparation of compound 1
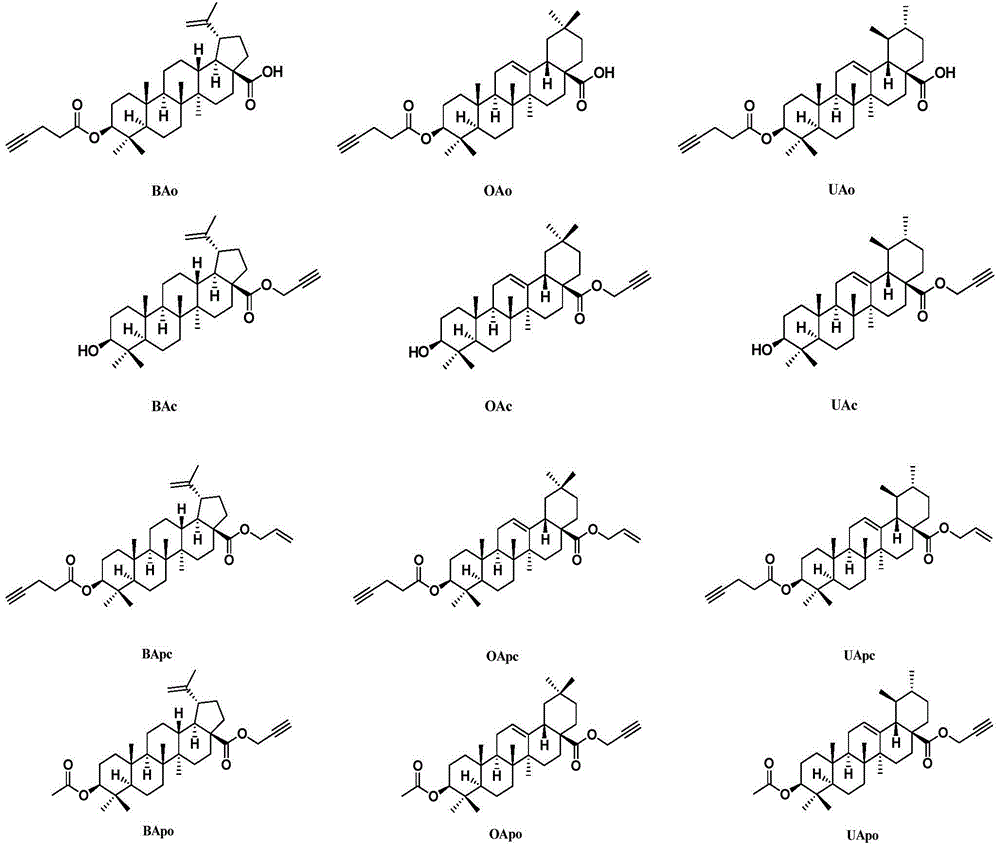
[0181] 1.1 Synthesis of small molecule compound BAc
[0182]
[0183] Synthesis of Intermediate 2:
[0184] Under nitrogen protection, 0.3 g (0.66 mmol) of compound 1, 0.11 g of imidazole and 8 mg of DMAP were dissolved in 2 ml of DMF. TBDPSCl 0.23 mL (0.79 mmol) was added. Stir at reflux overnight. After the reaction was completed, 25 mL of DCM was added, and the organic phase was washed with 1N hydrochloric acid and saturated sodium chloride solution, and dried over anhydrous sodium sulfate. Separation and purification by column chromatography gave a white solid with a yield of 72%.
[0185] Synthesis of intermediate 3:
[0186] 0.25 g of Intermediate 2 and 40 mg of DMAP were dissolved in 10 mL of DCM, and 0.11 g of pentynoic acid was added. Add 0.23g DIC under stirring, and continue stirring at room temperature until the reaction is complete. Add 25mL of DCM to dilute, wash with 10% citric acid, saturated sodium...
Embodiment 2-6
[0202] Embodiment 2-6: Preparation of Compound 2-3, 43-45
[0203] The method is the same as in Example 1, except that the BAc conjugated to the N-terminal of the polypeptide is replaced by UAc or OAc, or the N-terminal of the polypeptide is introduced into Ile to obtain compounds 2-3, 43-45.
Embodiment 7
[0204] Embodiment 7: the preparation of compound 4
[0205] 7.1 Synthesis of Small Molecule Compound BAO
[0206]
[0207] Weigh 0.5 g of compound 1 and dissolve it in 10 mL of DMF, add 0.46 g of potassium carbonate and 171 μl of propyne bromide. Stir at room temperature for 4 hours, and monitor the reaction by TLC. After the reaction was completed, the filtrate was filtered to remove salt and the filtrate was evaporated to dryness, followed by separation and purification by column chromatography to obtain a white solid with a yield of 65%.
[0208] 7.2 Preparation of BAc-peptide conjugate (compound 4): The synthesis process was the same as that of compound 1, except that the small molecule at the N-terminal of the peptide chain was replaced with BAo.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com