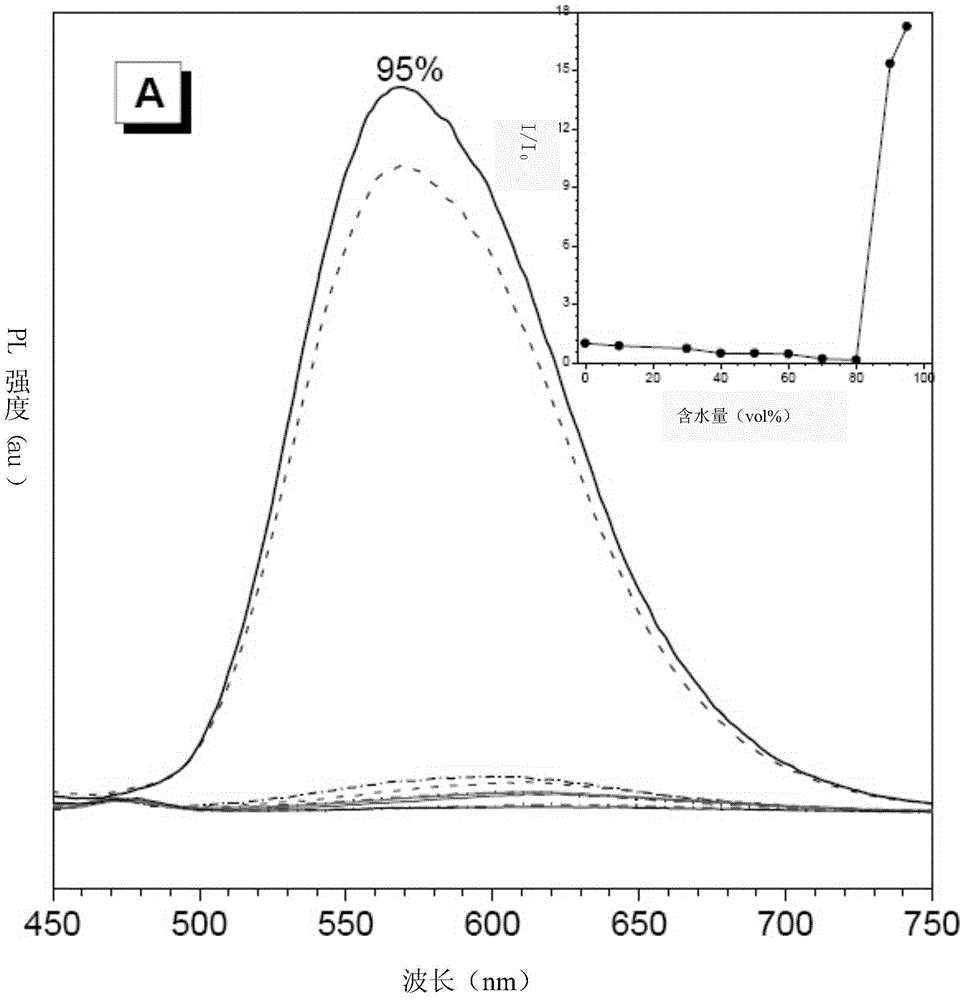
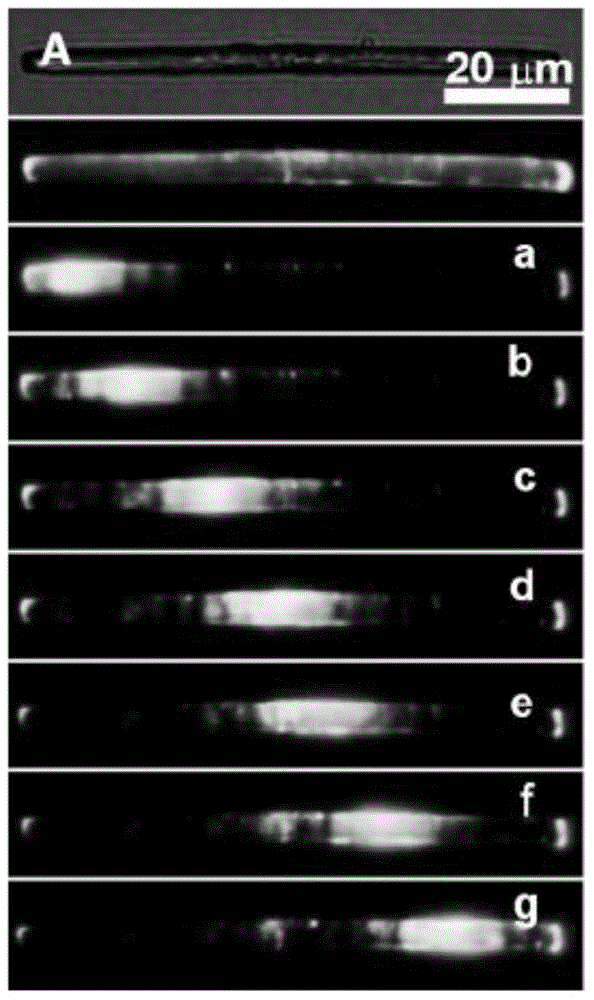
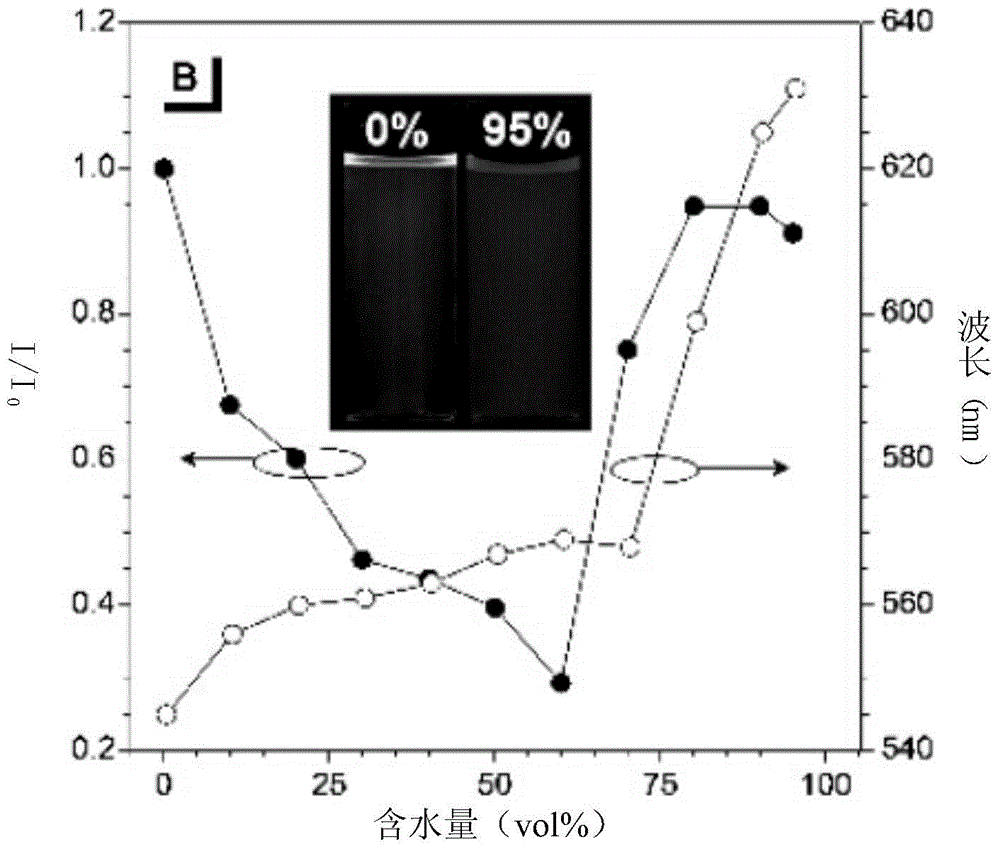
Luminescent material having aggregation-induced emission, method of making and application thereof
A technology of aggregation-induced luminescence and luminescence materials, applied in the field of solid-state luminescence materials, can solve problems such as limited application of luminescence materials, and achieve the effect of low light loss coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: Synthesis of TPE-Bar
[0065] Structural formula:
[0066]
[0067]Chinese chemical name: 5-(4-(1,2,2-triphenylethenyl)benzylidene)pyrimidine-2,4,6(1H,3H,5H)-trione
[0068] English chemical formula: 5-(4-(1,2,2-triphenylvinyl)benzylidene)pyrimidine-2,4,6(1H,3H,5H)-trione
[0069] Synthetic method: Mix 4-(1,2,2-triphenylvinyl)benzaldehyde 1 (500mg, 1.39mmol) and barbituric acid 3 (186mg, 1.46mmol) in a mixed solvent of methanol 20mL and THF 5mL After reflux for 24 hours, a bright yellow precipitate was formed. After the reaction mixture was cooled to room temperature, the formed bright yellow precipitate was filtered. The filtered solid was washed three times with ethanol and ether, respectively, and dried in vacuo to obtain 390 mg of the product with a yield of 61%. 1 H-NMR (400MHz, d 6 -DMSO): δ11.48(s, 1H), 11.33(s, 1H), 8.26(s, 1H), 8.11(d, J=8.8Hz, 2H), 7.32-7.23(m, 9H), 7.17( d,J=8.4Hz,2H),7.15-7.09(m,6H); 13 C-NMR (100MHz, d 6 -DMSO):163.44...
Embodiment 2
[0070] Embodiment 2: Synthesis of TPE-s-Bar
[0071] Structural formula:
[0072]
[0073] Chinese chemical name: 1,3-dimethyl-5-(4-(1,2,2-triphenylethenyl)benzylidene)pyrimidine-2,4,6(1H,3H,5H)- Triketone
[0074] English chemical formula: 1,3-dimethyl-5-(4-(1,2,2-triphenylvinyl)benzylidene)pyrimidine-2,4,6(1H,3H,5H)-trione
[0075] Synthetic method: Add 4-(1,2,2-triphenylvinyl)benzaldehyde 1 (500mg, 1.39mmol) and N,N-dimethylbarbituric acid 2 (227mg, 1.46mmol) to the mixture A drop of concentrated sulfuric acid was used as a catalyst, and refluxed in ethanol (20mL) for 24h to form an orange-yellow precipitate. After the reaction mixture was cooled to room temperature, the formed orange-yellow precipitate was filtered. The filtered solid was rinsed three times with ethanol and ether, and dried in vacuo. 480 mg of the product was obtained with a yield of 70%. HR-MS (MALDI-TOF) C 33 h 26 N 2 o 3 Theoretical value: 498.1943; Measured value: 498.1941; 1 H-NMR (400MHz,...
Embodiment 3
[0076] Embodiment 3: Synthesis of TPE-HPh-Bar
[0077] Structural formula:
[0078]
[0079] The steps include: (1) synthesizing TPE-HPh
[0080] Chinese chemical name: 2,5-dihexyloxy-4-((4-(1,2,2-triphenylethenyl)phenyl)ethynyl)benzaldehyde
[0081] English chemical formula: 2,5-dihexyloxy-4-((4-(1,2,2-triphenylvinyl)phenyl)ethynyl)benzaldehyde
[0082] Synthetic method: Add 2-(4-ethynylphenyl)-1,1,2-triphenylethenyl 4 (1g, 2.81mmol), 4-bromo-2,5- Dihexyloxybenzaldehyde 6 (1.03g, 2.68mmol), bis(triphenylphosphine)palladium(II) dichloride (Pd(PPh 3 ) 2 Cl 2 ) (0.089g, 0.126mmol), cuprous iodide (0.024g, 0.121mmol) and triphenylphosphine (0.066g, 0.251mmol), the above mixture was vacuumized and gas exchanged three times with nitrogen gas to completely remove oxygen, Pour 30 mL of newly distilled tetrahydrofuran and 10 mL of anhydrous degassed triethylamine into the above-mentioned reaction flask, and react at reflux for 8 hours at 70°C to form a precipitate. After cool...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com