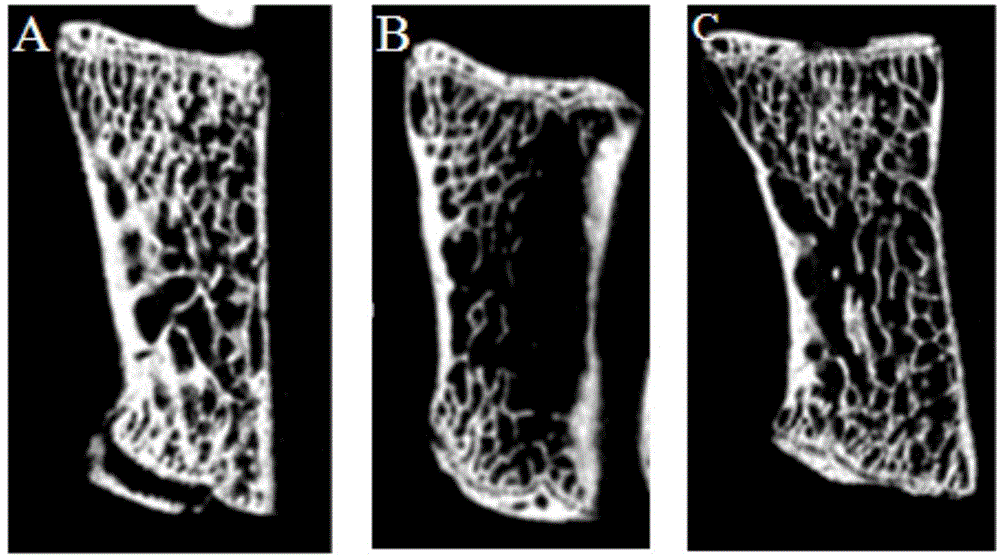
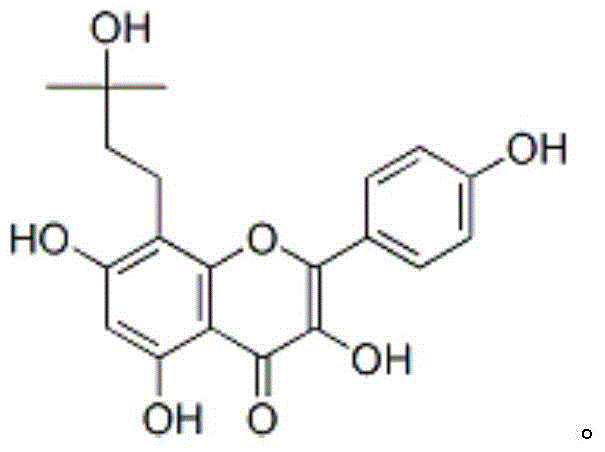
Pharmaceutical composition for treating osteoporosis, liposome preparation of pharmaceutical composition, and preparation method thereof
A technology for osteoporosis and composition, which is applied in the field of pharmaceutical composition for treating osteoporosis and its liposome preparation and preparation, can solve the problem that calcium preparation cannot be effectively absorbed and utilized, slow-release effect is not good, stable The problem of poor sexuality, etc., to achieve exact therapeutic effect, moderate effect, and small side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] One embodiment of the present invention provides a method for preparing liposome preparations, comprising: dissolving 10-100 parts by mass of egg yolk lecithin and cholesterol in a mixed solvent of chloroform and methanol, wherein the above-mentioned egg yolk lecithin and cholesterol The mass ratio is 2:1-6:1; then the chloroform and methanol are evaporated under reduced pressure to form a uniform lipid film; 1-5 mass parts of icariin, 1-5 mass parts of glycosides, 1 -5 parts by mass of loganin are dissolved in phosphate buffer, and then the buffer is added to the above lipid film as the water phase, and then several beads are added to shake until the film falls off completely, and then placed in a water bath at 20-80°C Swell in medium for 5-30min, then stir at a constant speed for 5-30min, ultrasonicate in an ice bath for 5-60min to obtain a liposome suspension, and then squeeze the above liposome suspension through a microporous membrane , to obtain liposome preparati...
Embodiment 1
[0049] The liposome preparation of this embodiment is prepared by thin film dispersion method. Dissolve 4 g of yolk lecithin and cholesterol for injection (W / W, 4:1) in an appropriate amount of chloroform and methanol (V / V, 1:1), and then use a rotary evaporator to evaporate under reduced pressure to remove chloroform and methanol to make the lipid The substance forms a uniform lipid film on the wall of the pear-shaped bottle; weigh 0.3g icariin, 0.3g and loganin 0.3g and dissolve them in a phosphate buffer (10mL) with a pH of 7.2, and then The buffer solution (10 mL) was added to the lipid film as the water phase, and then ten glass beads (5 mm in diameter) were added, oscillated until the film fell off completely, swelled in a water bath at 40°C for 10 min, and then stirred at a constant speed for 10 min. Sonicate in the bath for 30 minutes to obtain a liposome suspension, and then squeeze it through microporous membranes with pore diameters of 0.80 micron, 0.45 micron, and ...
Embodiment 2
[0051] The liposome preparation of this embodiment is prepared by thin film dispersion method. Dissolve 3 g of egg yolk lecithin for injection and cholesterol (W / W, 2:1) in an appropriate amount of chloroform and methanol (V / V, 3:1), and then use a rotary evaporator to evaporate under reduced pressure to remove chloroform and methanol to make the lipid The substance forms a uniform lipid film on the wall of the pear-shaped bottle; Weigh 0.1g of icariin, 0.1g of icaritin and 0.1g of loganin and dissolve it in a phosphate buffer (5mL) with a pH of 7.2, and then The buffer solution (5 mL) was added to the lipid film as the water phase, and then ten glass beads (particle diameter: 2 mm) were added, oscillated until the film fell off completely, swelled in a water bath at 80°C for 5 min, and then stirred at a constant speed for 5 min. Sonicate in the bath for 60 minutes to obtain a liposome suspension, which is then squeezed through microporous membranes with pore diameters of 0.80...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com