A kind of preparation method of composite photocatalyst
A catalyst and composite light technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve problems such as no literature reports, and achieve the effect of easy drying and removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 5,000 mg of melamine into a crucible, cover the crucible, put it into an intelligent programmable muffle furnace, heat from room temperature to 550°C, set the heating rate to 4°C / min, and continue heating at 550°C for 3 hours, then Cool naturally to room temperature to obtain g-C 3 N 4 . Prepare a 5 wt % aqueous solution of hydrazine hydrate, then add 500 mg of g-C under magnetic stirring 3 N 4 After stirring for 10 minutes, 200 mg of ferric nitrate was added, and stirring was continued for 20 minutes; the mixture was put into an oven and heated at 100° C. for 15 hours to obtain the final product.
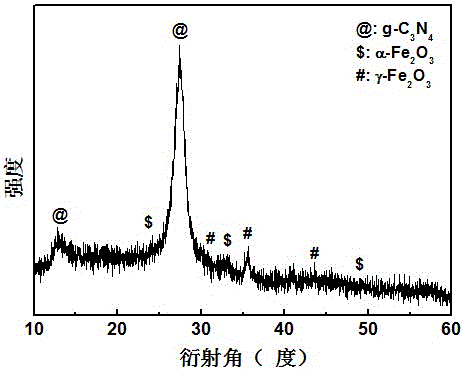
[0027] Adopt German Bruker-AXS company D8 ADVANCE polycrystalline X-ray diffractometer, analyze the phase of the product made, such as figure 1 shown. The result shows: the product made in embodiment 1 is g-C 3 N 4 , α-Fe 2 o 3 and γ-Fe 2 o 3 compound.
Embodiment 2
[0029] Weigh 5,000 mg of melamine into a crucible, cover the crucible, put it into an intelligent programmable muffle furnace, heat from room temperature to 550°C, set the heating rate to 4°C / min, and continue heating at 550°C for 3 hours, then Cool naturally to room temperature to obtain g-C 3 N 4 ; Prepare 5wt% aqueous solution of hydrazine hydrate, then add 500 mg of g-C under magnetic stirring 3 N 4 After stirring for 10 minutes, 300 mg of ferric nitrate was added, and stirring was continued for 20 minutes; the mixture was put into an oven and heated at 100° C. for 15 hours to obtain the final product.
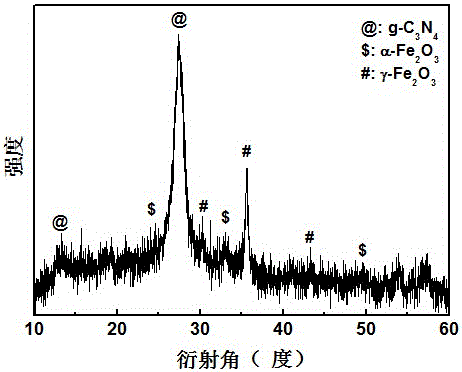
[0030] Adopt German Bruker-AXS company D8 ADVANCE polycrystalline X-ray diffractometer, analyze the phase of the product made, such as figure 2 shown. The result shows: the product made by embodiment 2 is g-C 3 N 4 , α-Fe 2 o 3 and γ-Fe 2 o 3 compound.
Embodiment 3
[0032] Weigh 5,000 mg of melamine into a crucible, cover the crucible, put it into an intelligent programmable muffle furnace, heat from room temperature to 550°C, set the heating rate to 4°C / min, and continue heating at 550°C for 3 hours, then Cool naturally to room temperature to obtain g-C 3 N 4 ; prepare a 5 wt % aqueous solution of hydrazine hydrate, then add 500 mg of g-C under magnetic stirring 3 N 4 After stirring for 10 minutes, 400 mg of ferric nitrate was added, and stirring was continued for 20 minutes; the mixture was put into an oven and heated at 100° C. for 15 hours to obtain the final product.
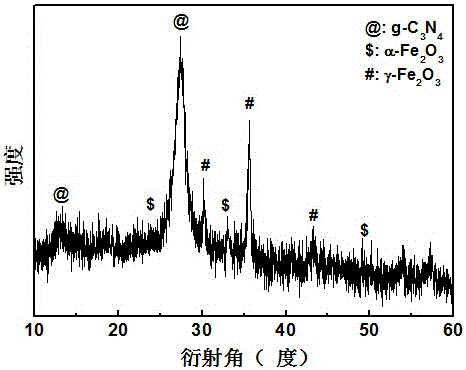
[0033] Adopt German Bruker-AXS company D8 ADVANCE polycrystalline X-ray diffractometer, analyze the phase of the product made, such as image 3 shown. The result shows: the product made by embodiment 3 is g-C 3 N 4 , α-Fe 2 o 3 and γ-Fe 2 o 3 compound.
[0034] 2. Test the photocatalytic performance of the above composite materials:
[0035] The photocataly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com