Preparation method of chirality 2-methyl cysteine and hydrochloride thereof
A technology of methylcysteine and hydrochloride, which is applied in the field of preparation of chiral 2-methylcysteine and its hydrochloride, can solve the difficulty in purification of methylated products, which cannot meet the requirements of industrial production, Low production efficiency and other issues, to achieve the effect of high product purity and optical purity, good industrial application value, and improved production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
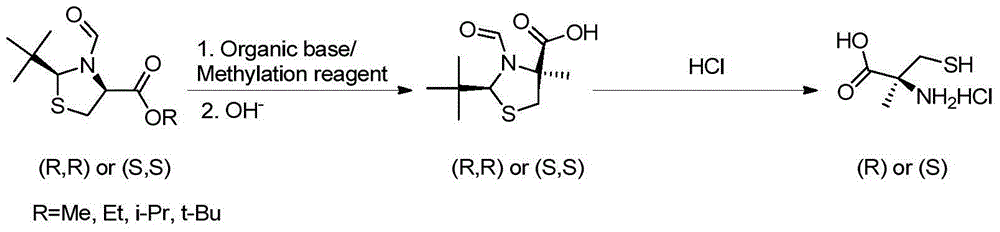
[0023] Example 1 Preparation of 2-methyl-D-cysteine and its hydrochloride
[0024] A) (2S,4S)-2-tert-butyl-3-formyltetrahydrothiazole-4-methyl-4-carboxylic acid
[0025] At -90°C, (2S,4S)-2-tert-butyl-3-formyltetrahydrothiazole-4-carboxylic acid methyl ester (23.1g, 0.1mol) was dissolved in anhydrous THF ( 230ml), under nitrogen protection, add iodomethane (28.6g, 0.20mol) under stirring, and keep warm for 10min after the dropwise addition. Slowly add NaHMDS (22 g, 0.20 mol) in batches, and keep the reaction for 2 h after the addition is complete.
[0026] At room temperature, add 1N aqueous sodium hydroxide solution (100ml) to the reaction system, stir overnight, add 500ml of water, extract with ethyl acetate (300ml*3), separate the organic layer, add 1N hydrochloric acid dropwise to the aqueous layer until pH = 4. Filter to obtain a white solid. The white solid was recrystallized in toluene to obtain 20 g of white solid, yield: 87%.
[0027] B) 2-Methyl-D-cysteine hy...
Embodiment 2
[0031] Example 2 Preparation of 2-methyl-L-cysteine and its hydrochloride
[0032] A) (2R,4R)-2-tert-butyl-3-formyltetrahydrothiazole-4-methyl-4-carboxylic acid
[0033] At -90°C, (2R,4R)-2-tert-butyl-3-formyltetrahydrothiazole-4-carboxylic acid methyl ester (23.1g, 0.1mol) was dissolved in anhydrous THF ( 230ml), and methyl iodide (28.6g, 0.20mol) was added with stirring. Slowly add NaHMDS (22 g, 0.2 mol) in batches, and keep the reaction for 2 hours after the addition is complete.
[0034] At room temperature, 1N aqueous sodium hydroxide solution (100 ml) was poured into the reaction system, and left overnight at room temperature. Add 500ml of water and extract with ethyl acetate (300ml*3). 1N hydrochloric acid was added dropwise to the aqueous layer until pH = 4, a large amount of white solids precipitated, and the white solids were obtained by filtration. The white solids were recrystallized in toluene to obtain 20.8 g of white solids, yield: 90%.
[0035] B) 2-Methy...
Embodiment 3
[0039] Example 3 Preparation of 2-methyl-D-cysteine and its hydrochloride
[0040] A) (2S,4S)-2-tert-butyl-3-formyltetrahydrothiazole-4-methyl-4-carboxylic acid
[0041] At -40°C, (2S,4S)-2-tert-butyl-3-formyltetrahydrothiazole-4-carboxylic acid methyl ester (23.1g, 0.1mol) was dissolved in anhydrous THF ( 230ml), under nitrogen protection, add iodomethane (28.6g, 0.20mol) under stirring, and keep warm for 10min after the dropwise addition. Slowly add LiHMDS (22 g, 0.20 mol) in batches, and keep the reaction for 2 h after the addition is complete.
[0042] At room temperature, add 1N aqueous sodium hydroxide solution (100ml) to the reaction system, stir overnight, add 500ml of water, extract with ethyl acetate (300ml*3), separate the organic layer, add 1N hydrochloric acid dropwise to the aqueous layer until pH= 4. Filter to obtain a white solid. The white solid was recrystallized in toluene to obtain 16 g of white solid, yield: 70%.
[0043] B) 2-Methyl-D-cysteine hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com