Rhizopus microsporus root-shaped variant ZJPH1308 and application thereof in preparation of sitagliptin intermediate
A kind of technology of Rhizopus microspora and varieties, applied in the field of application of Rhizopus microspora whisker variety ZJPH1308 and in the preparation of sitagliptin intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the acquisition of wet thalline
[0055] The composition of PDA medium is: potato 200g / L, glucose 20g / L, agar 20g / L, solvent is distilled water, pH is natural;
[0056] The formula of the seed medium is as follows: glucose 25.0g / L, peptone 27.5g / L, ammonium sulfate 3.0g / L, potassium dihydrogen phosphate 1.0g / L, sodium chloride 0.3g / L, distilled water, pH 6.0.
[0057] The formula of the fermentation medium is as follows: dextrin 25.0g / L, beef extract 30.0g / L, ammonium sulfate 5.0g / L, calcium chloride 0.4g / L, sodium chloride 0.3g / L, cobalt chloride 0.05g / L , prepared in distilled water, pH 6.0.
[0058] Inoculate Rhizopus microsporin varietus ZJPH1308 into PDA medium, and culture at 30°C for 2-3 days; then transfer the mature slant strains into a 250mL shake flask containing 100mL seed medium, culture at 30°C, 200rpm After 22 hours, the seed liquid was transferred to a 250 mL shake flask containing 100 mL of fermentation liquid with an inoculum volume con...
Embodiment 2
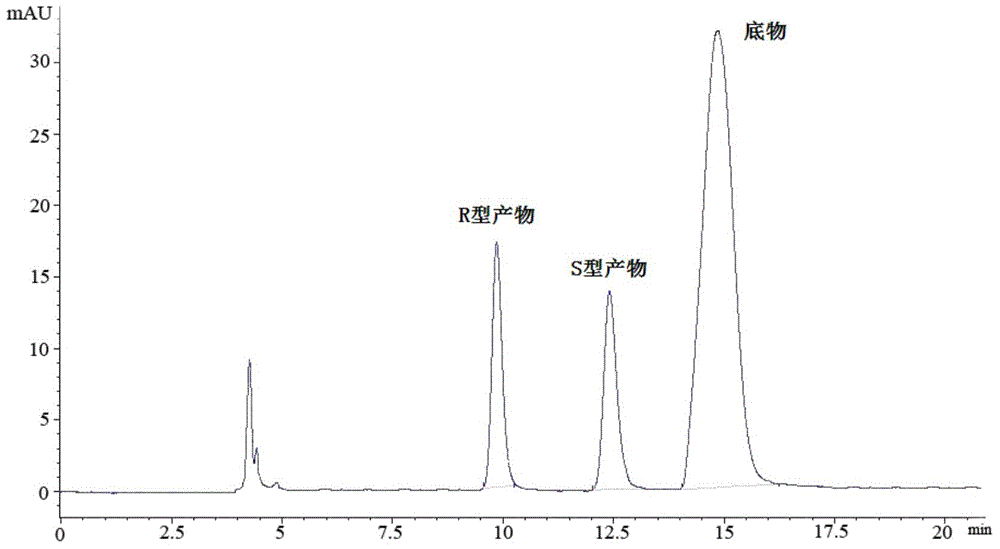
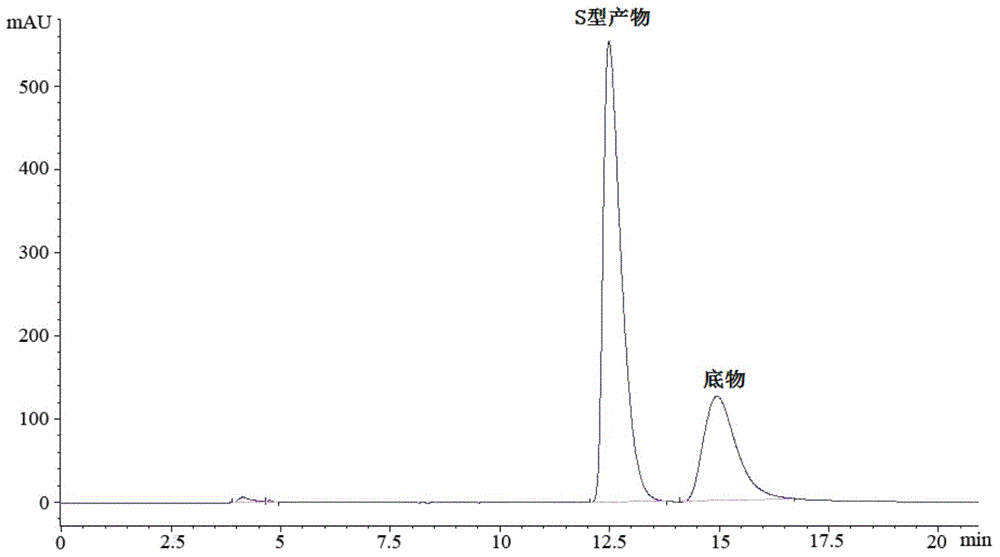
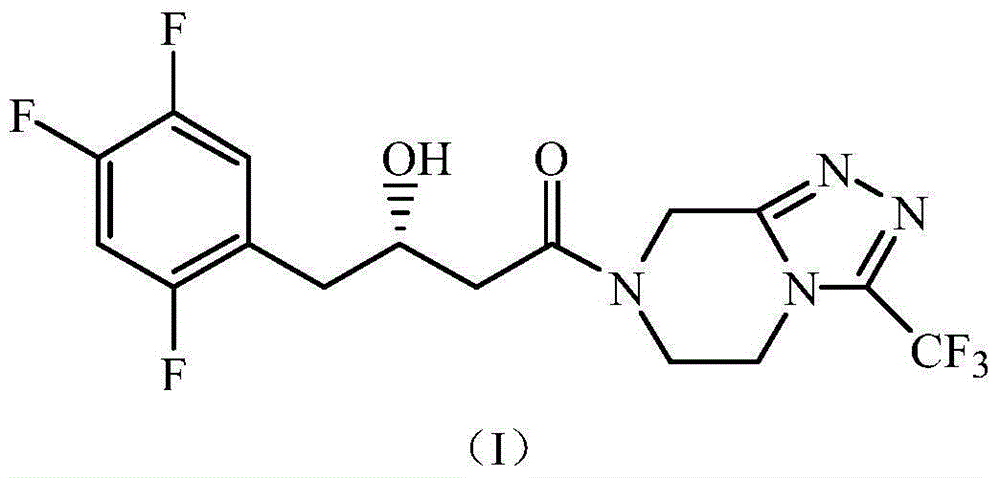
[0060] The wet bacterium obtained in Example 1 was resuspended in 100mmol / L, pH 6.0 phosphate buffer, the wet bacterium was 350g / L in terms of buffer volume, and 4-oxo-4-[ 3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7-(8H)-yl]-1-(2, 4,5-trifluorophenyl)butan-2-one substrate, and adding 6% (v / v) of glycerol in the volume of the reaction medium as an auxiliary substrate, 30°C, 200rpm, shaking the reaction for 24h. Detected by liquid chromatography, the product (S)-3-hydroxyl-1-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3- a] The concentration of pyrazin-7-(8H)-yl]-4-(2,4,5-trifluorophenyl)butan-1-one is 0.233mmol / L, the e.e. value is >99.9%, and the yield is 23.3%. The liquid chromatograms of substrate and product standards are shown in figure 1 See figure 2 ;product 1 H-NMR nuclear magnetic resonance spectrum result is as follows: 1 H-NMR (400MHz.DMSO-d 6 )δ / ppm: 7.46-7.41(q,2H), 5.02-4.83(m,3H), 4.22(s,1H), 4.09(s,2H), 4.02-3.90(m,2H), 2.78-2.68...
Embodiment 3
[0063] The phosphate buffer of 100mmol / L, pH 6.0 among the embodiment 2 is replaced with distilled water of the same volume, other operation is the same as embodiment 2, product (S)-3-hydroxyl-1-[3-(trifluoromethyl)- 5,6-Dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7-(8H)-yl]-4-(2,4,5-trifluorophenyl) The concentration of butan-1-one was 0.801 mmol / L, the e.e. value was >99.9%, and the yield was 80.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com