High affinity adapter body capable of specifically binding with saxitoxin acetate and application thereof
A saxitoxin and aptamer technology, applied in the biological field, can solve the problems of affinity constant detection, optimization and application that have not yet been reported, 4-aminopyridine has large toxic and side effects, and the safe dose range is small.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. Aptamer APT STX1 mutation
[0029] First, we send the APT STX1 A single point mutation is introduced into the sequence. We mutated the nucleotides at either end of the guanine nucleotides in the sequence to guanine nucleotides. By this method, we obtained 22 single-point mutation oligonucleotide sequences, the mutation positions are 3, 6, 8, 12, 13, 15, 20, 22, 25, 30, 32, 37, 41, 43, 48, 50, 55, 57, 58, 60, 61 and 64. According to the prediction results of QGRS Mapper software, there are 9 sequences (M-6, M-8, M-12, M-13, M-15, M-20, M-22, M-30 and M-32) may form a G-quadruplex structure. We synthesized the above nine sequences and evaluated their STX binding ability by biomembrane interferometry (see Table 1). Similarly, we also introduced multiple point mutations, replacing two or three nucleotides near guanine nucleotides with guanine nucleotides, and synthesized 6 sequences that may form a G-quadruplex structure, And evaluate its STX binding abili...
Embodiment 2
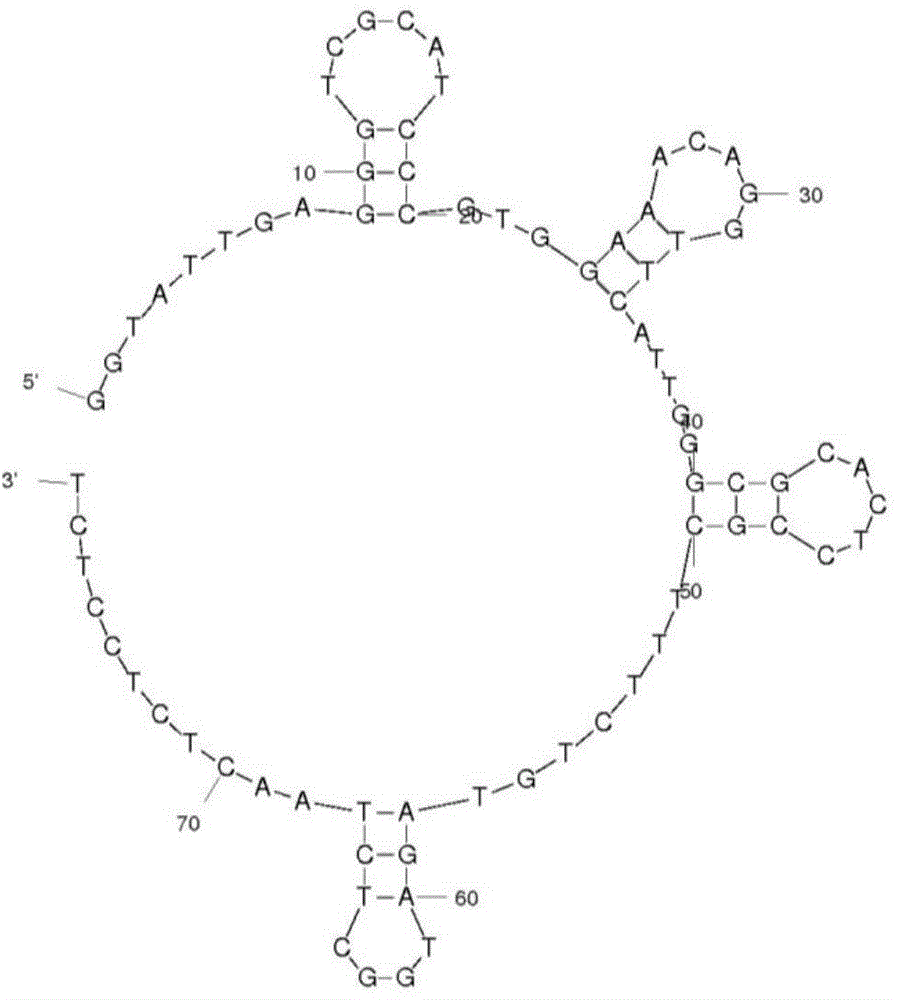
[0040] Example 2. Truncation optimization of aptamers
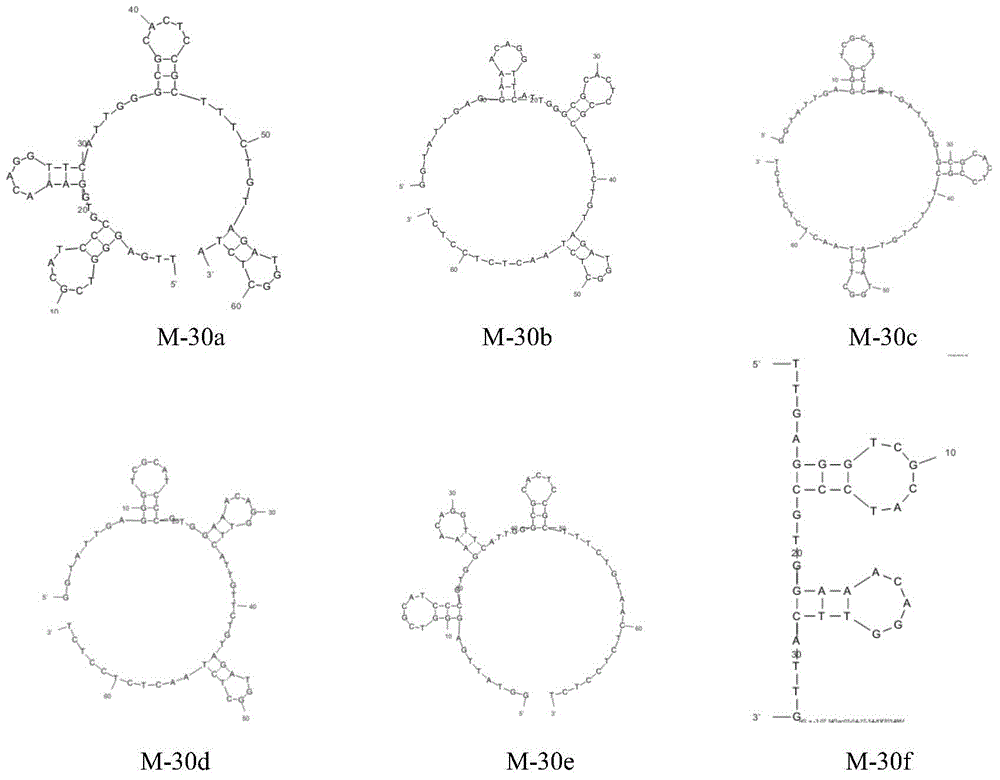
[0041] Using mfold software, we predicted the secondary structure of M-30, such as figure 1 shown. By removing the single-stranded sequences at both ends of M-30 or any of the four stem-loop structures, we obtained five sequences (M-30a, M-30b, M-30c, M-30d, M- 30e; if figure 2 ), were synthesized and their Kd values were determined by biofilm interferometry (Table 3).
[0042] Among the five sequences, M-30a, M-30d and M-30e respectively remove the free nucleotides at both ends, the third stem-loop and the fourth stem-loop structure. The Kd values for sequence M-30 are very close. However, after removing the first or second stem-loop structure (M-30b, M-30c), the affinity of the aptamer decreased significantly. This indicates that the first and second stem-loop structures are critical for target molecule recognition. Based on these results and our reasoning, we synthesized M-30f by removing the third and fourt...
Embodiment 3
[0045] Example 3. The affinity and specificity of M-30f binding to STX were analyzed by biofilm interference technology
[0046] After coupling 1 μM M-30f to the streptavidin-coated chip surface by using the function of biotin-streptavidin, it interacted with different concentrations of STX (5 μM, 10 μM, 20 μM) and obtained corresponding responses Curve, use the data analysis software to adopt the 1:1 binding mode to fit the response curves of different concentrations of STX, and obtain the binding and dissociation curves of the interaction between the two, such as image 3 , Kd value is 133nM. In addition, no response was observed when GTX2,3 (20 μM) was used as the target molecule for analysis by biofilm interferometry. This indicates that M-30f does not cross-react with GTX2,3 and retains its specificity for STX.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com