UPLC (ultra-high performance liquid chromatography) method for simultaneously determining six related substances in bicalutamide
A technology for bicalutamide and related substances, which is applied in the detection field of chemical drug related substances, can solve problems such as less bicalutamide, and achieve the effects of ensuring safe medication, high sensitivity and shortening analysis time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
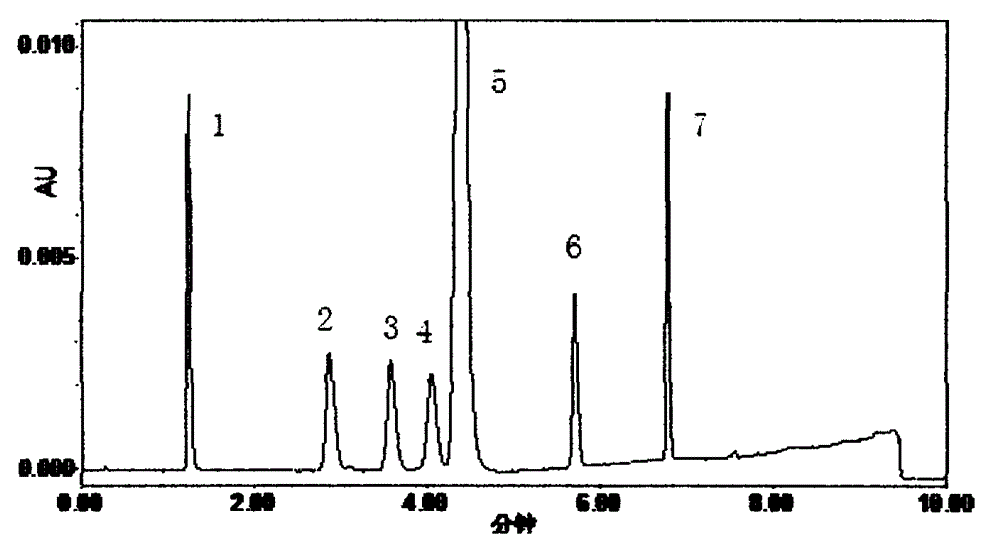
[0015] The determination of 6 kinds of related substances in the bicalutamide crude drug of embodiment 1
[0016] 1 Instruments and reagents
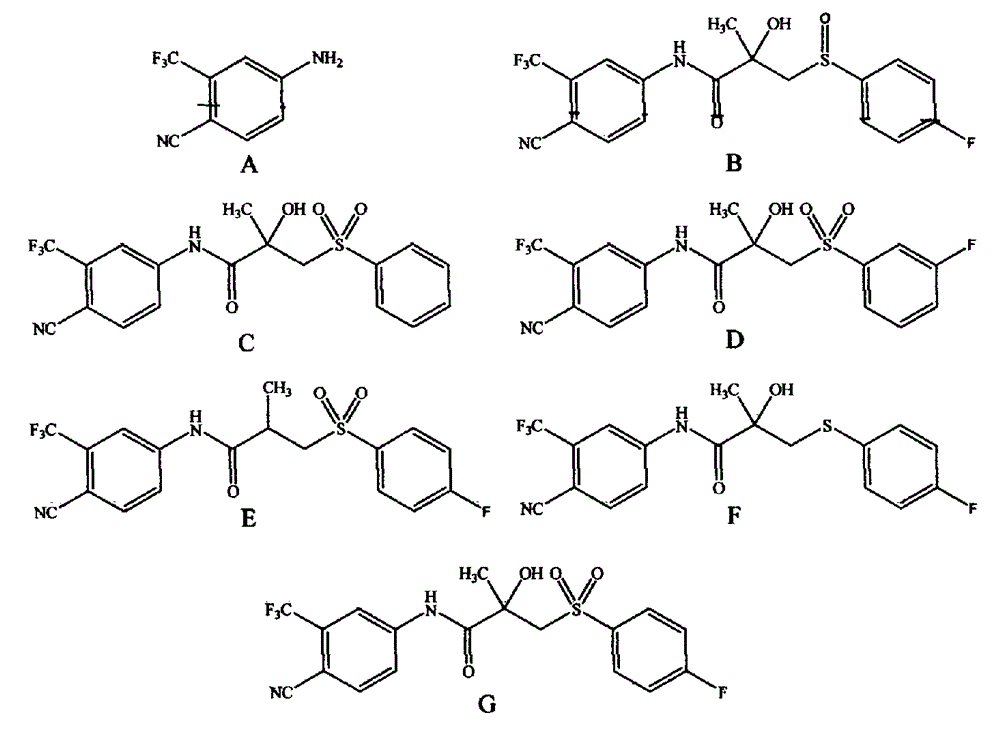
[0017] Waters ACQUITY ultra-high performance liquid chromatography, the reagents used are chromatographically pure, and the water is ultrapure water. Three batches of bicalutamide API, each batch 46g; bicalutamide reference substance 200mg; 4-amino-2-(trifluoromethyl)benzonitrile reference substance 20mg; impurity A reference substance 20mg; Reference substance 20mg; 2-fluoroisomer reference substance 20mg; dehydroxy homologue reference substance 20mg; bicalutamide sulfide reference substance 20mg.
[0018] 2 Methods and results
[0019] 2.1 Chromatographic conditions and system suitability
[0020] Column: ACQUITY UPLC BEH C 18 (1.7 μ m, 2.1 * 50mm); Mobile phase A is the aqueous solution of 0.01% (v / v) trifluoroacetic acid, and mobile phase B is the acetonitrile solution of 0.01% (v / v) trifluoroacetic acid, carries out gradient wa...
Embodiment 2
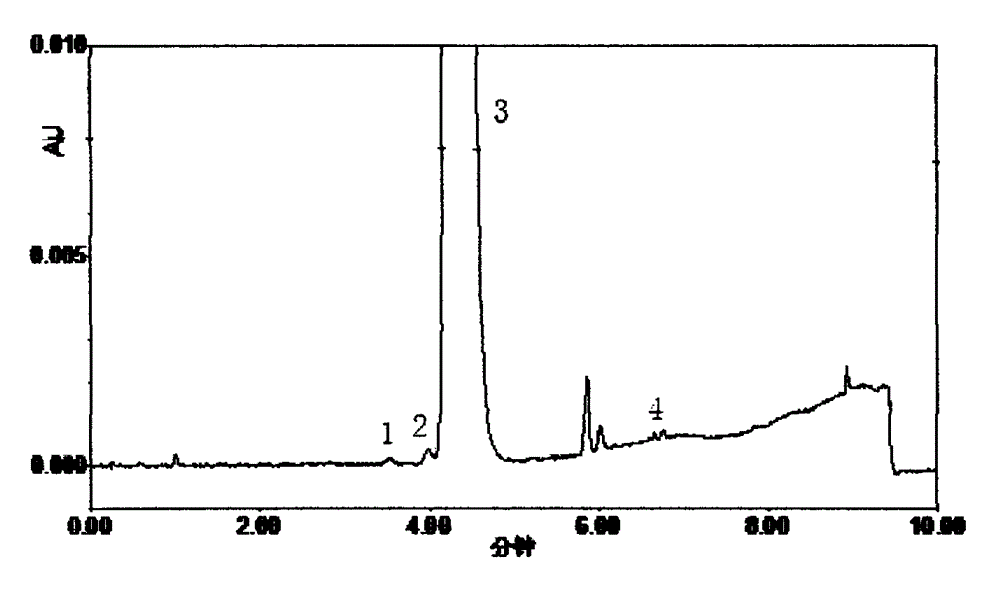
[0040] Determination of 6 kinds of related substances in embodiment 2 bicalutamide tablets
[0041] 2.7 Sample Determination
[0042] Take three batches of 20 bicalutamide tablets (specification: 50mg / tablet) with known content, weigh them accurately, grind them finely in a mortar, weigh an appropriate amount, add a diluent and ultrasonically dissolve them for 30 minutes, and quantitatively dilute them to make each 1mL Contain about 1 mg of the solution in the solution, shake well, filter with a microporous membrane (0.22 μm), and take the subsequent filtrate to obtain the test solution. Take an appropriate amount of bicalutamide reference substance, accurately weigh it, dissolve it with a diluent and quantitatively dilute it to make a solution containing about 1 μg per 1 mL, as the reference substance solution. Precisely measure 1.5 μL of the reference substance solution and inject it into the liquid chromatograph, adjust the detection sensitivity so that the peak height of ...
Embodiment 3
[0046] Determination of 6 kinds of related substances in embodiment 3 bicalutamide capsules
[0047] 2.7 Sample Determination
[0048] Take the contents of 20 bicalutamide capsules (specification: 50mg / capsule) of three batches of known content respectively, weigh them accurately, take an appropriate amount, add diluent and ultrasonically dissolve them for 30min, and quantitatively dilute them to make about Containing 1 mg of the solution, shake well, filter through a microporous membrane (0.22 μm), and take the subsequent filtrate to obtain the test solution. Take an appropriate amount of bicalutamide reference substance, accurately weigh it, dissolve it with a diluent and quantitatively dilute it to make a solution containing about 1 μg per 1 mL, as the reference substance solution. Precisely measure 1.5 μL of the reference substance solution and inject it into the liquid chromatograph, adjust the detection sensitivity so that the peak height of the main component chromatog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com