Alkaline phosphatase activity assay method based on in-situ formation of optical active nanometer material mimic enzyme
A nanomaterial, phosphatase technology, applied in the field of nanotechnology and biological analysis and detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
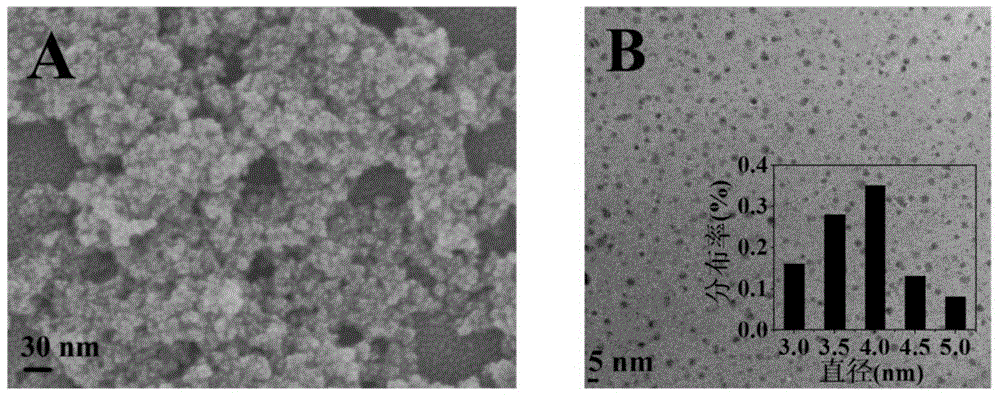
[0016] a. Add 5 mL of tetrabutyl titanate dropwise into 75 mL of ultrapure water, and keep stirring the mixed solution at room temperature. The above solution was then transferred into a reaction kettle and heated to 160 °C for 24 h. The product was obtained by centrifugation and washed several times with ultrapure water, and finally, dried to obtain white titanium dioxide nanoparticles;
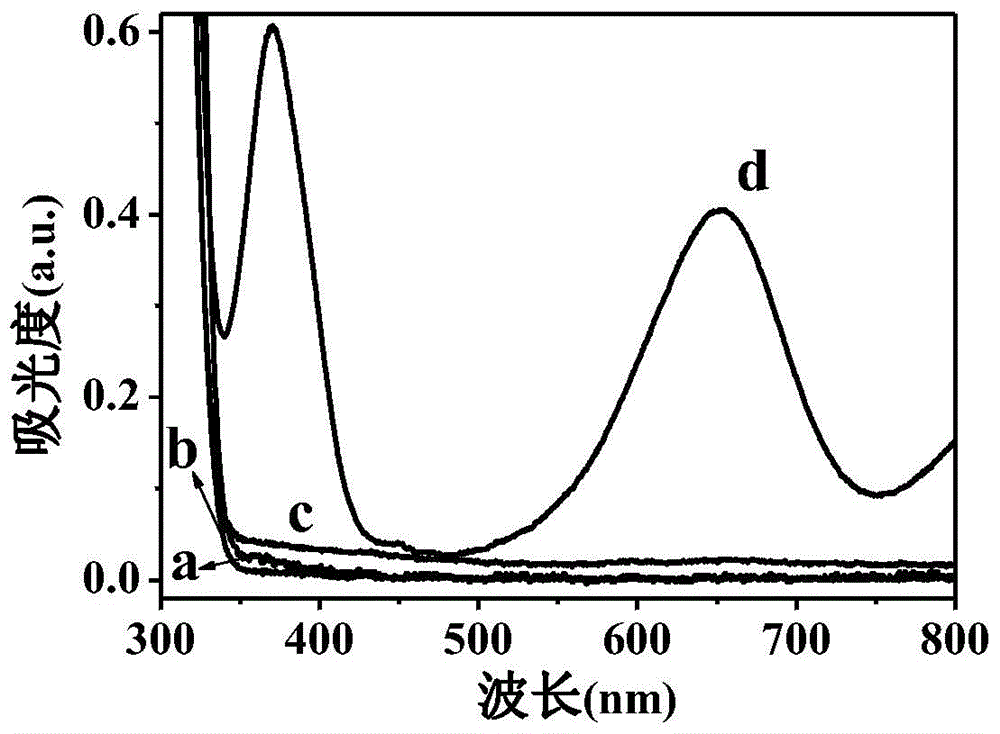
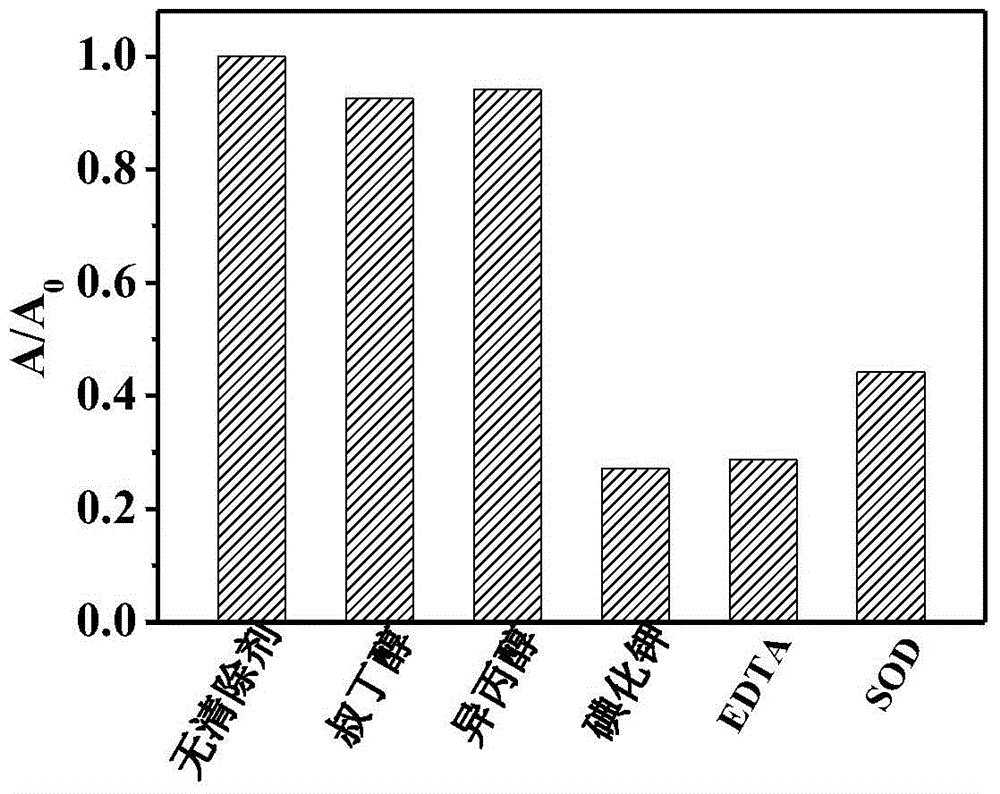
[0017] b. 5 μmol / L catechol phosphate was mixed with 10 μL alkaline phosphatase of different concentrations and added to a 96-well plate, and reacted at room temperature for 1 h; after adding 10 μL 1.5 mg / mL titanium dioxide nanomaterials and reacted for 15 min, Add 100 μL of 0.2 mol / L acetate buffer solution with a pH of 4.0 and 20 μL of 5 mmol / L characteristic substrate 3,3',5,5'-tetramethylbenzidine, place it under a xenon lamp with visible light (λ≥400nm) After irradiating for 10min, the characteristic absorption (λ max =652nm) to measure the absorption spectrum.
Embodiment 2
[0019] a. Add 5 mL of titanium tetrachloride dropwise to 75 mL of ultrapure water, and keep stirring the mixed solution at room temperature. The above solution was then transferred into a reaction kettle and heated to 160 °C for 24 h. The product was obtained by centrifugation and washed several times with ultrapure water, and finally dried to obtain white titanium dioxide nanoparticles;
[0020]b. 5 μmol / L salicylic acid phosphate was mixed with 10 μL alkaline phosphatase of different concentrations and added to a 96-well plate, and reacted at room temperature for 1 hour; 100 μL of 0.2 mol / L acetate buffer solution with a pH of 4.0 and 20 μL of 5 mmol / L characteristic substrate 2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, After placed under a xenon lamp and irradiated with visible light (λ≥400nm) for 10 minutes, the characteristic absorption of the oxidation product of 2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt ( lambda ...
Embodiment 3
[0022] a. Add 5 mL of tetrabutyl titanate dropwise into 75 mL of ultrapure water, and keep stirring the mixed solution at room temperature. The above solution was then transferred into a reaction kettle and heated to 160 °C for 24 h. The product was obtained by centrifugation and washed several times with ultrapure water, and finally dried to obtain white titanium dioxide nanoparticles;
[0023] b. 5 μmol / L catechol phosphate was mixed with 10 μL alkaline phosphatase of different concentrations and added to a 96-well plate, and reacted at room temperature for 1 h; after adding 10 μL 1.5 mg / mL titanium dioxide nanomaterials and reacted for 15 min, Add 100 μL of 0.2 mol / L acetate buffer solution with pH 4.0 and 20 μL of 5 mmol / L characteristic substrate 2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt , placed under a xenon lamp and irradiated with visible light (λ≥400nm) for 10 minutes, the characteristic absorption of the oxidation product of 2,2'-azinobi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com