Coumarin derivative and preparation method thereof
A technology of coumarin derivatives and methyl coumarin, which is applied in the field of coumarin derivatives and their preparation, can solve the problems of limited application scope, poor insecticidal effect, poor solubility, etc. Insecticidal effect, good biological activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
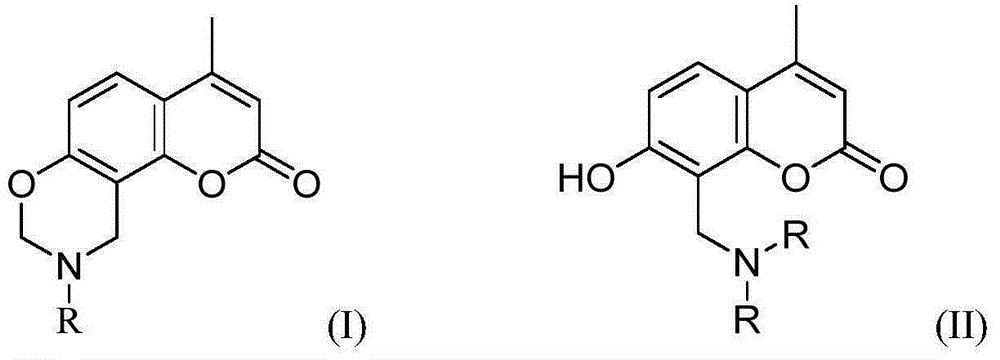
[0035] The preparation of embodiment 1 7-hydroxy-4-methylcoumarin
[0036] In a 250ml three-necked flask equipped with a magnetic stirrer, a thermometer and a constant pressure dropping funnel, add resorcinol (11.0g, 100.0mmol) and ethyl acetoacetate (12.6ml, 100.0mmol), stir well until dissolved, and place on ice Cool the water bath to 0°C, slowly add concentrated sulfuric acid (1.0g, 10.2mmol) dropwise, react at 0°C for 30min, then slowly raise the temperature to room temperature and react for 90min, follow the reaction by TLC, and pour the reaction solution into 200.0ml of ice In water, stir the ice-water mixture evenly, let it stand for 10 minutes, vacuum filter to obtain the crude product of 7-hydroxy-4-methylcoumarin, and then recrystallize through industrial alcohol to obtain 16.3g of white needle-shaped product, which is 7-hydroxy-4-methylcoumarin 4-methylcoumarin, yield 92.5%. The qualitative characterization data of gained 7-hydroxyl-4-methylcoumarin is: 1 H-NMR (5...
Embodiment 2
[0037] The preparation of embodiment 2 coumarin derivatives
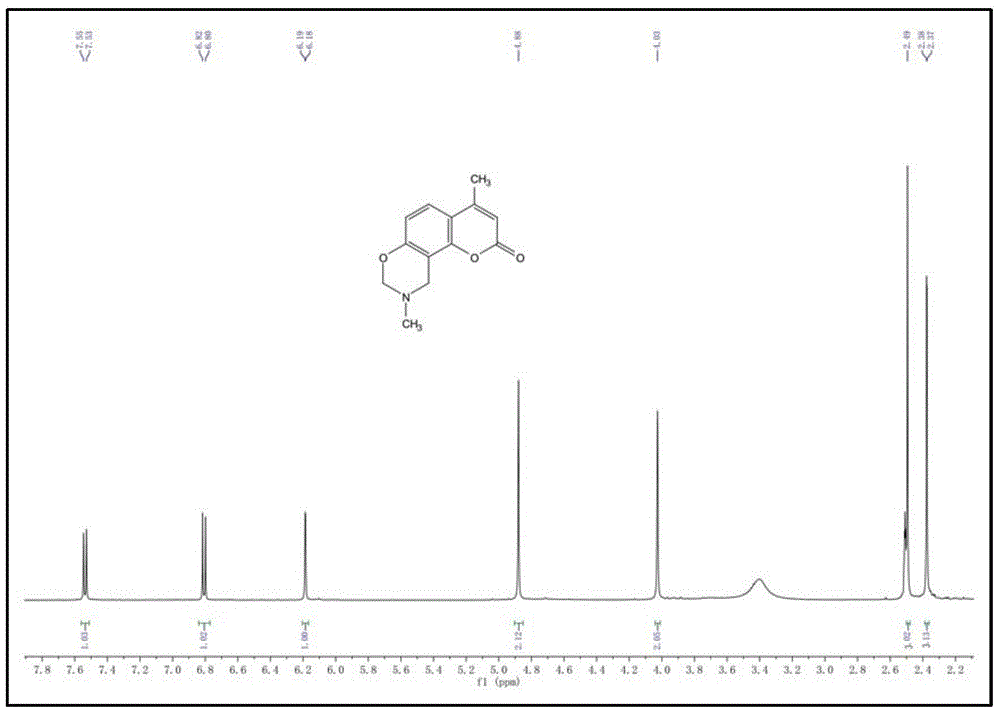
[0038] Add 7-hydroxy-4-methylcoumarin (1.7g, 10.0mmol) and industrial ethanol (15.0ml) into a 25ml single-necked flask equipped with a magnetic stirrer and stir well at room temperature until dissolved, then add 40.0% methylamine in mass fraction Aqueous solution (1.1ml, 10.0mmol) and 37.5% formaldehyde solution (1.5ml, 20.0mmol) were slowly warmed up to 80°C, refluxed for 5 hours, and the end point of the reaction was determined by TLC. The reaction solvent was removed by rotary evaporation to obtain an oily substance, and water (20.0ml) was added to solidify the oily substance, and then the crude product of yellow coumarin derivative was obtained by suction filtration, washed three times with water (60ml), and then recrystallized through absolute ethanol to obtain 1.9g The light yellow product is the desired coumarin derivative ① with a yield of 82.1%. The structural formula of compound ① is:
[0039]
[0040...
Embodiment 3-9
[0041] The preparation of embodiment 3-9 coumarin derivatives
[0042] Using the same operating steps and the same amount of 7-hydroxyl-4 methylcoumarin, industrial ethanol, formaldehyde and organic primary or secondary amines in Example 2, different types of organic primary or secondary amines, different reaction temperatures and times, to prepare fragrances Soybean derivatives, the experimental data obtained are as shown in table 1:
[0043] Table 1:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com