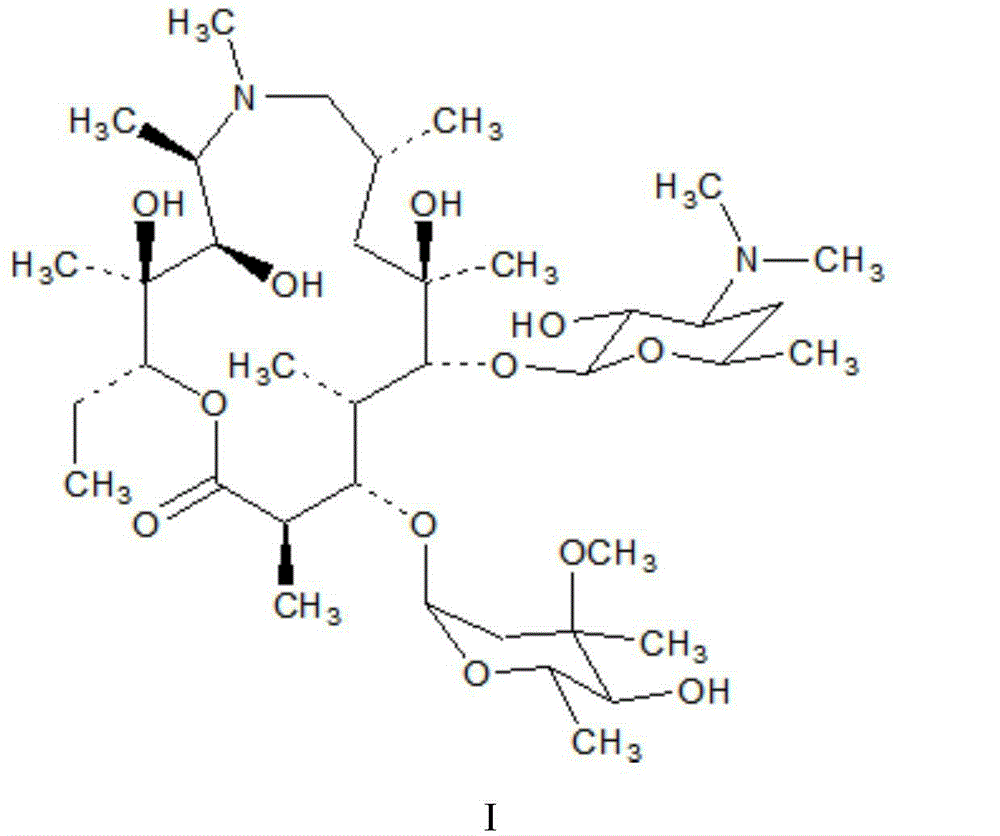
Azithromycin new crystal-form compound and preparation method thereof
A technology of azithromycin and compounds, applied in the field of medicine, can solve the problems of low stability of azithromycin crystalline products, difficulty in solvent recovery, poor fluidity, etc., and achieve good quality stability, good fluidity, and suitable for long-term storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
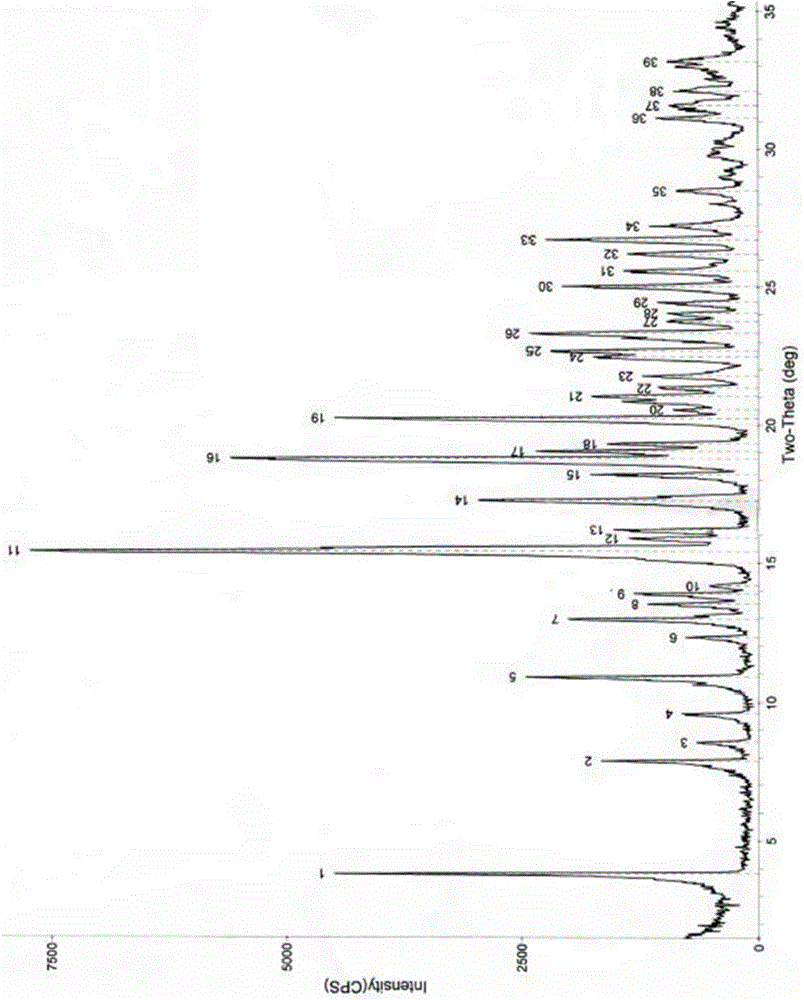
[0028] Example 1 Preparation of Azithromycin New Crystal Form Compound
[0029] Add 375ml of methyl acetate and 625ml of isopropyl ether to 100g of crude azithromycin, heat to 50°C to dissolve, cool to room temperature, then add 500mL of water to the solution, stir and mix evenly, then cool down to 5-10°C, and stand for crystallization After 3 hours, it was filtered and dried to obtain 97.7 g of the new crystal form of azithromycin, with a yield of 97.7% and an HPLC purity of 99.82%.
Embodiment 2
[0030] Example 2 Preparation of Azithromycin New Crystal Form Compound
[0031] Add 570ml of methyl acetate and 1430ml of isopropyl ether to 100g of crude azithromycin, heat to 40°C to dissolve, cool to room temperature, then add 200mL of water to the solution, stir and mix evenly, then cool down to 5-10°C, and stand for crystallization After 5 hours, it was filtered and dried to obtain 95.1 g of the new crystal form of azithromycin, with a yield of 95.1% and an HPLC purity of 99.52%.
Embodiment 3
[0032] Example 3 Preparation of Azithromycin New Crystal Form Compound
[0033] Add 35ml of methyl acetate and 165ml of isopropyl ether to 100g of crude azithromycin, heat to 45°C to dissolve, cool to room temperature, then add 200mL of water to the solution, stir and mix evenly, stand for crystallization for 1h, filter, and dry to obtain azithromycin The new crystalline compound was 98.4g, the yield was 98.4%, and the HPLC purity was 99.43%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com