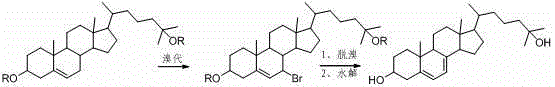
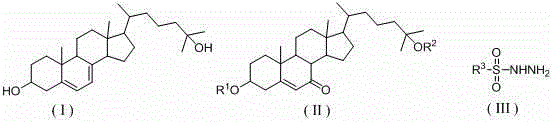
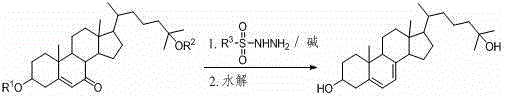
25-hydroxy-7-dehydrocholesterol synthetic method
A technique for the synthesis of dehydrocholesterol, which is applied in the fields of steroids and organic chemistry, can solve problems such as cumbersome steps, and achieve the effects of high reaction efficiency, good reaction selectivity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] At room temperature, 4.86 g (10 mmol) of 25-hydroxycholesterol diacetate (R 1 =R 2 =Ac), 2.05g (11 mmol) p-toluenesulfonyl hydrazide, 1.19g (22 mmol) sodium methoxide were successively added into the ball milling tank, and the ball milling reaction was carried out for 0.5 hours. After the reaction, the reaction mixture was washed and filtered with 20 mL of water, and the filtered Add the cake to 25mL ethanol (95%) to dissolve, add sodium hydroxide, control the pH value ≥ 13, react at 50°C for 1 hour, cool down to room temperature to crystallize, filter to obtain a light yellow solid, and then recrystallize with ethanol to obtain a white tablet Crystalline, 25-hydroxy-7-dehydrocholesterol 3.52g, yield 88%, melting point 191~193℃, 1 H NMR (400 MHz, CDCl 3 ) δ = 5.55 - 5.57 (m, 1H), 5.36 - 5.39 (m, 1H), 3.59-3.67 (m, 1H), 2.44 - 2.49 (m, 1H), 2.25 - 2.31(m, 1H), 2.06 - 2.11(m, 1H), 1.87 - 1.99(m, 4H), 1.54 - 1.75 (m, 7H), 1.26 - 1.51 (m, 11H), 1.22 (s, 6H), 1.02 - 1.10 ...
Embodiment 2
[0024] At room temperature, 4.86 g (10 mmol) of 25-hydroxycholesterol diacetate (R 1 =R 2 =Ac), 2.05g (11 mmol) p-toluenesulfonyl hydrazide, 0.18g (22 mmol) lithium hydride were successively added into the ball milling tank, and the ball milling reaction was carried out for 0.5 hours. After the reaction, the reaction mixture was washed and filtered with 20 mL of water, and the filtered Add the cake to 25mL ethanol (95%) to dissolve, add sodium hydroxide, control the pH value ≥ 13, react at 50°C for 1 hour, cool down to room temperature to crystallize, filter to obtain a light yellow solid, and then recrystallize with ethanol to obtain a white tablet 25-hydroxy-7-dehydrocholesterol 3.48g, yield 87%, melting point 191~193℃.
Embodiment 3
[0026] At room temperature, 4.86 g (10 mmol) of 25-hydroxycholesterol diacetate (R 1 =R 2 =Ac), 2.05g (11 mmol) p-toluenesulfonyl hydrazide, 0.86g (22 mmol) sodium amide were successively added to the ball milling tank, and the ball milling reaction was 0.5 hours. After the reaction, the reaction mixture was washed and filtered with 20 mL of water, and the filtered Add the cake to 25mL ethanol (95%) to dissolve, add sodium hydroxide, control the pH value ≥ 13, react at 50°C for 1 hour, cool down to room temperature to crystallize, filter to obtain a light yellow solid, and then recrystallize with ethanol to obtain a white tablet 25-hydroxy-7-dehydrocholesterol 3.56g, yield 89%, melting point 191~193℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com