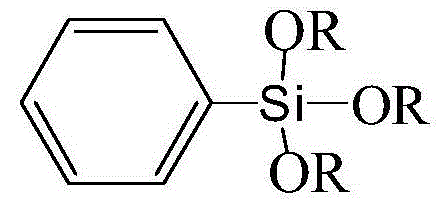
Method for preparing phenyl trialkyl alkoxy silane by nucleophilic method
A technology of phenyltrialkoxysilane and tetraalkoxysilane, which is applied in the field of synthesis of organosilicon compounds, can solve the problems of extremely high requirements for equipment drying, unfavorable large-scale production, and high risk of reaction, and achieve the elimination of unnecessary Potential safety hazards, moderate and controllable production conditions, and the effect of less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Under the protection of nitrogen, add 23g of sodium block into 92g of toluene, then add 0.46g of palmitic acid, and heat to 102°C to prepare metal sodium particle suspension. Add the metal sodium particle suspension to the mixed solution of 63.75g chlorobenzene and 345g tetramethoxysilane, then add 0.43g cyclodextrin, react at 40°C for 5h, stop the reaction, and filter the reaction mixture, Distillation under reduced pressure gave phenyltrimethoxysilane with a yield of 86.5%.
Embodiment 2
[0032] Under nitrogen protection, add 46g of sodium block to 230g of toluene, then add 0.77g of paraffin, heat to 107°C to prepare a suspension of metallic sodium particles, lower the temperature to 40°C, add 120.79g of chlorobenzene dropwise to metallic sodium In the particle suspension, after 3 hours of reaction, a phenyl sodium suspension is generated, and then the phenyl sodium suspension is added dropwise to a mixture of 1230.2g tetraethoxysilane and 1.37g 15-C-5, at 40°C , reacted for 4h, stopped the reaction, filtered the reaction mixture, and rectified under reduced pressure to obtain phenyltriethoxysilane with a yield of 78%.
Embodiment 3
[0034] Under the protection of nitrogen, add 72g of sodium block to 504g of toluene, then add 1.26g of oleic acid, heat to 112°C to prepare metal sodium particle suspension, lower the temperature to 45°C, add 172.13g of chlorobenzene dropwise to the metal In the sodium particle suspension, react for 2 hours to form a phenyl sodium suspension, and then add the phenyl sodium suspension dropwise to a mixture of 1630.3g tetramethoxysilane and 1.63g 18-C-6, at 45°C , reacted for 2.5 hours, stopped the reaction, filtered the reaction mixture, and rectified under reduced pressure to obtain phenyltrimethoxysilane with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com