Technology for preparing iron phosphate through hydrolytic method
A technology of iron phosphate and hydrolysis method, applied in phosphorus compounds, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of high energy consumption, ammonia nitrogen pollution, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The first step is oxidation. Get 278g (1mol) ferrous sulfate heptahydrate (100%) into a beaker, add 600ml of water to dissolve, then take 115.3g (1mol) of phosphoric acid with a mass percentage of 85%, add it to the ferrous sulfate solution, stir evenly, and , 90ml (0.8mol) of 27.5% hydrogen peroxide was added dropwise in ten minutes to oxidize ferrous iron into ferric iron. After adding the hydrogen peroxide, add water to make the volume of the solution 1 L. Heat the above materials to 90-95°C;
[0022] The second step is hydrolysis. In a reaction kettle with a capacity of 20L, add 10L of deionized water and heat to 90-95°C. The solution obtained in the first step is quickly added into the hot water in the second step under stirring, and the concentration of the hydrolyzate is 0.09 mol / L (calculated as Fe). Due to the rapid decrease in pH value, hydrolysis occurs, resulting in iron phosphate precipitation;
[0023] The third step is separation. Immediately while ...
Embodiment 2
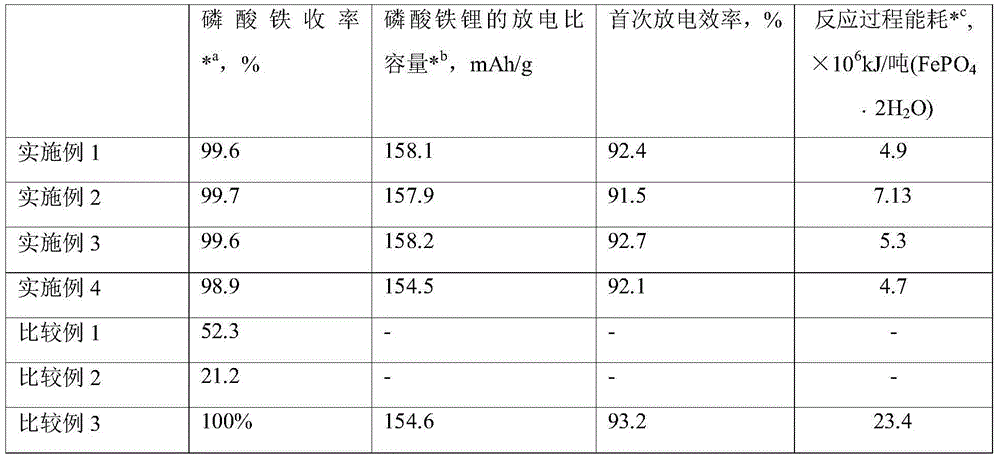
[0027] The second step hydrolysis water consumption in embodiment 1 is brought up to 15L, and the concentration of hydrolyzate is 0.03mol / L (calculated as Fe), other is with embodiment 1. The results are shown in Table 1.
Embodiment 3
[0029] The volume of the water of the dissolved material in the first step in Example 1 is doubled. After adding the hydrogen peroxide, add water to make the volume of the solution 2L, that is, the amount of raw materials remains unchanged and the volume increases to 2L. Others are the same as in Example 1. . The concentration of the hydrolyzate is 0.08mol / L (calculated as Fe), and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com