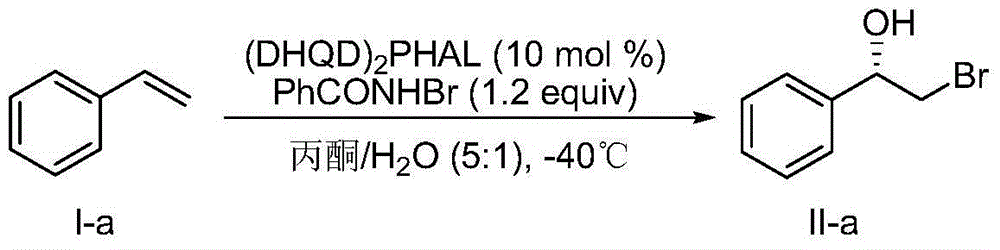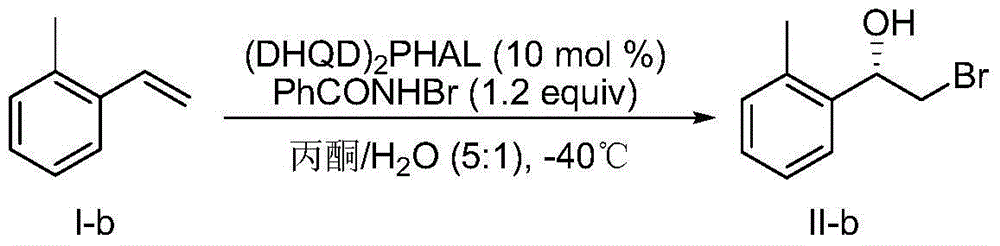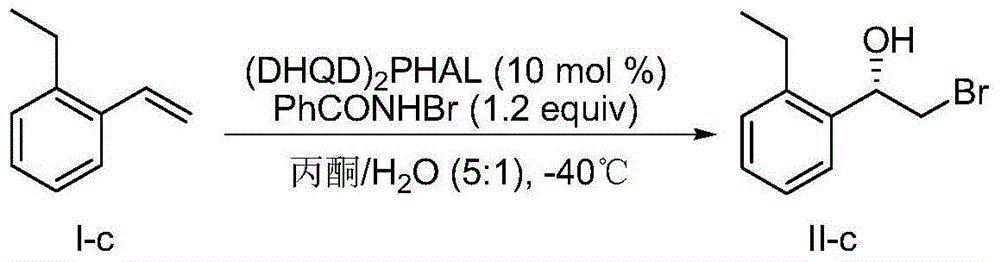Hydroxyl bromine compound preparation method
A compound and catalyst technology, applied in the field of preparation of hydroxybromide compounds, can solve problems such as complex preparation process, and achieve the effects of easy availability of raw materials, mild reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, preparation (S)-2-bromo-1-phenylethanol:
[0046] according to figure 1 The flowchart in the preparation:
[0047] In test tube add (DHQD) 2 PHAL (0.0389g, 0.05mmol) and N-bromobenzamide (0.120g, 0.6mmol), dissolved in 5mL of acetone and 1mL of water, stirred at -40°C for 15min, then added the compound shown in formula I-a (0.0521g, 0.5mmol), react at -40°C for 72h, add 10mL saturated sodium thiosulfate solution to quench, separate the layers, extract the aqueous phase with dichloromethane (10mL x 3), dry over anhydrous magnesium sulfate, filter, and concentrate by rotary evaporation , Purified by silica gel column chromatography, the yield is 64%, and it is known by HPLC test that one enantiomer in the product is 60% more than the other enantiomer (ie, the enantiomeric excess is 60%).
[0048] HPLC conditions: chiral OD-H column; the volume ratio of n-hexane: isopropanol is 95:5; flow rate: 1.0mL / min; absorption wavelength: 210nm.
[0049] The results...
Embodiment 2
[0051] Embodiment 2, preparation (S)-2-bromo-1-(2-methylphenyl)ethanol:
[0052] according to figure 2 The flowchart in the preparation:
[0053] Add in test tube (DHQD) 2PHAL (0.0389g, 0.05mmol) and N-bromobenzamide (0.120g, 0.6mmol), dissolved in 5mL acetone and 1mL water, stirred at -40°C for 15min, then added formula I-b (0.0591g, 0.5mmol) , reacted at -40°C for 72h, quenched by adding 10mL saturated sodium thiosulfate solution, separated the layers, extracted the aqueous phase with dichloromethane (10mL x 3), dried over anhydrous magnesium sulfate, filtered, concentrated by rotary evaporation, and silica gel column Purified by chromatography, the yield was 78%, and the enantiomeric excess (measured by HPLC) was 83%.
[0054] HPLC conditions: chiral OD-H column; n-hexane:isopropanol volume ratio of 95:5; flow rate: 1.0mL / min; absorption wavelength: 220nm.
[0055] The results of structural confirmation are as follows: 1 H NMR (400Hz, CDCl 3 )δ7.42-7.30 (m, 5H), 4.93...
Embodiment 3
[0057] Example 3, preparation of (S)-2-bromo-1-(2-ethylphenyl)ethanol:
[0058] according to image 3 The flowchart in the preparation:
[0059] In test tube add (DHQD) 2 PHAL (0.0389g, 0.05mmol) and N-bromobenzamide (0.120g, 0.6mmol), dissolved in 5mL acetone and 1mL water, stirred at -40°C for 15min, then added formula I-c (0.0661g, 0.5mmol) , reacted at -40°C for 72h, quenched by adding 10mL saturated sodium thiosulfate solution, separated the layers, extracted the aqueous phase with dichloromethane (10mL x 3), dried over anhydrous magnesium sulfate, filtered, concentrated by rotary evaporation, and silica gel column Purified by chromatography, the yield was 68%, and the enantiomeric excess (measured by HPLC) was 71%.
[0060] HPLC conditions: chiral AD-H column; n-hexane:isopropanol volume ratio of 95:5; flow rate: 1.0mL / min; absorption wavelength: 220nm.
[0061] The results of structural confirmation are as follows: 1 H NMR (400Hz, CDCl3) δ7.55-7.47 (m, 1H), 7.30-7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com