Preparation method of anhydrous lanthanum chloride
A technology of lanthanum chloride and lanthanum carbonate, which is applied in the chemical field, can solve the problems of complex reaction process, low solubility of finished products, long production cycle, etc., and achieve the effect of controllable reaction process, simple reaction process and high dissolution rate of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
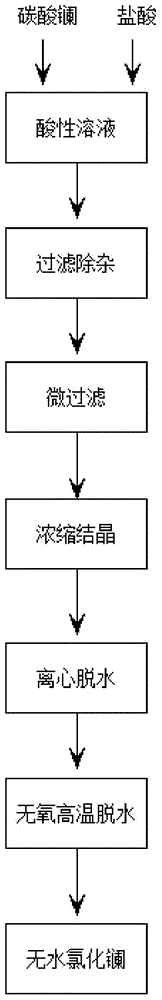
[0028] A preparation method for anhydrous lanthanum chloride, comprising the following steps:
[0029] (1), 1 part of lanthanum carbonate powder is dissolved with 2 parts of dilute hydrochloric acid to form an acidic solution, and then an appropriate amount of dilute hydrochloric acid is added dropwise to the acidic solution until the pH of the acidic solution is 2, and the reaction is stirred for 1 hour;
[0030] (2) Filtration and impurity removal
[0031] Obtain the filtrate after filtering the mixed solution that also has precipitation in the step (1) through a 1000-mesh sieve;
[0032] (3) microfiltration
[0033] The filtrate of step (2) is added into a microporous filter to obtain the filtrate after microfiltration;
[0034] (4) Evaporation and concentration
[0035] Heating the filtrate of step (3) to 150° C., and incubating for 5 hours to obtain a concentrated solution;
[0036] (5) cooling crystallization
[0037] Cool the concentrated solution of step (4) to 20...
specific Embodiment 2
[0044] A preparation method for anhydrous lanthanum chloride, comprising the following steps:
[0045] (1), 1 part of lanthanum carbonate powder was dissolved with 4 parts of dilute hydrochloric acid to form an acidic solution, then an appropriate amount of dilute hydrochloric acid was added dropwise to the acidic solution until the pH of the acidic solution was 3, and the reaction was stirred for 5 hours;
[0046] (2) Filtration and impurity removal
[0047] Obtain the filtrate after filtering the mixed solution that also has precipitation in the step (1) through a 1000-mesh sieve;
[0048] (3) microfiltration
[0049] The filtrate of step (2) is added into a microporous filter to obtain the filtrate after microfiltration;
[0050] (4) Evaporation and concentration
[0051] Heating the filtrate of step (3) to 150° C., and incubating for 7 hours to obtain a concentrated solution;
[0052] (5) cooling crystallization
[0053] Cool the concentrated solution of step (4) to 2...
specific Embodiment 3
[0060] A preparation method for anhydrous lanthanum chloride, comprising the following steps:
[0061] (1), 1 part of lanthanum carbonate powder was dissolved with 3 parts of dilute hydrochloric acid to form an acidic solution, then an appropriate amount of dilute hydrochloric acid was added dropwise to the acidic solution until the pH of the acidic solution was 2.5, and the reaction was stirred for 3 hours;
[0062] (2) Filtration and impurity removal
[0063] Obtain the filtrate after filtering the mixed solution that also has precipitation in the step (1) through a 1000-mesh sieve;
[0064] (3) microfiltration
[0065] The filtrate of step (2) is added into a microporous filter to obtain the filtrate after microfiltration;
[0066] (4) Evaporation and concentration
[0067] Heating the filtrate of step (3) to 150° C., and keeping it warm for 6 hours to obtain a concentrated solution;
[0068] (5) cooling crystallization
[0069] Cool the concentrated solution of step (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com