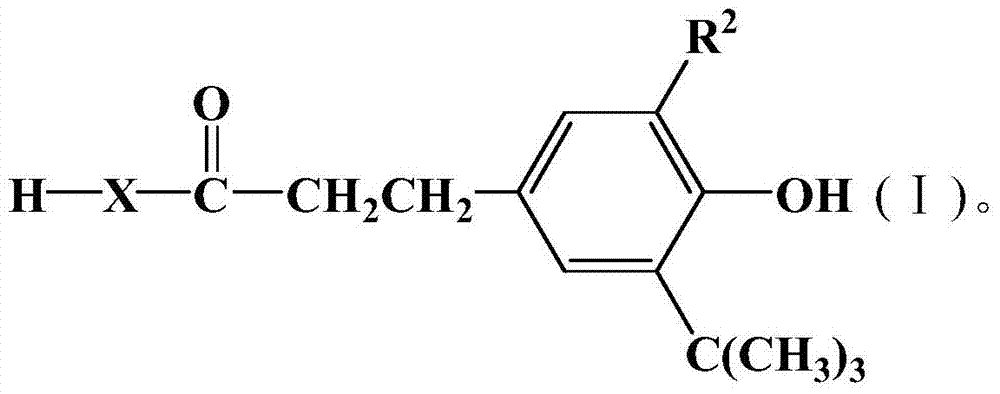
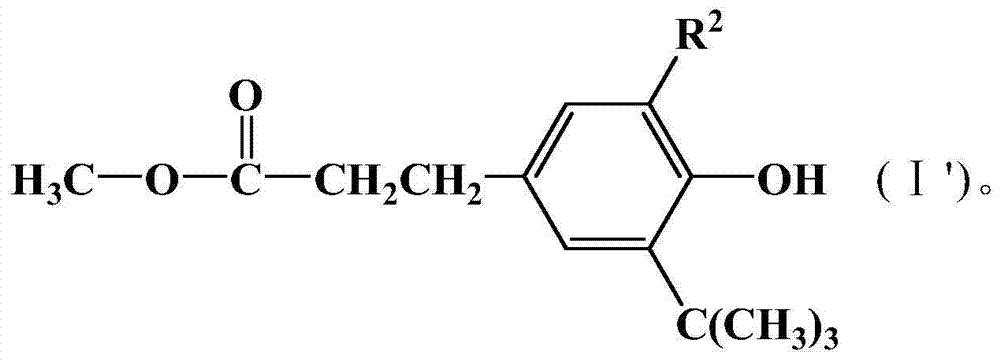
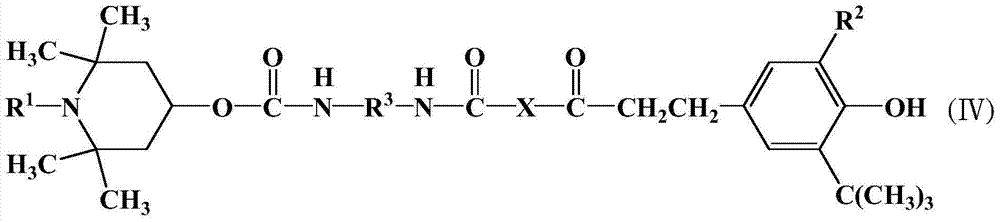Organic material stabilizer and modified polymer containing tetramethylpiperidinyl
A technology of tetramethylpiperidinyl and organic materials, which is applied in the direction of organic chemistry, can solve the problems such as the effect is not obvious, and achieve the effect of improving the effect of yellowing resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Bifunctional compound A
[0082] Add 250mL of ethanol, 60g of 80% hydrazine hydrate and 146g of methyl β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate into a 500mL four-neck mechanically stirred bottle, heat up to reflux for 6 hours, and detect with TLC When there was no methyl β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate, 150 mL of water was added to lower the temperature, and the product was crystallized and completely precipitated. After filtration and drying, 141 g of white solid powder was obtained.
[0083] Deoxygenate another 500mL four-neck mechanical stirring bottle with nitrogen, add 250mL toluene and 50g (0.2mol) 4,4'-methylenebis(phenylisocyanate) (MDI), and put it at 10-15℃ Slowly add 58.4g (0.2mol) of the above white solid powder first, there will be exothermic phenomenon during the addition process, so that the temperature will naturally rise to 40-45°C, and then add 31.4g (0.2mol) 2,2, 6,6-Tetramethyl-4-piperidinol (2,2,6,6-tetramethyl-4-piperidinol...
Embodiment 2
[0085] Bifunctional compound B
[0086] The synthetic method of embodiment 2 is the same as embodiment 1, and difference is that 33.6g1,6-hexamethylene diisocyanate (HDI) replaces MDI, obtains 112g white solid [being the difunctional compound shown in following formula (B) B], the yield is 90.8%.
[0087]
Embodiment 3
[0088] Bifunctional compound C
[0089] The synthesis method of embodiment 3 is the same as that of embodiment 1, except that 146g beta-(3,5-di- Methyl tert-butyl-4-hydroxyphenyl)propionate, after reacting with hydrazine hydrate in ethanol, gave 101 g of an intermediate product. Get 50g (0.2mol) of above-mentioned intermediate product and MDI and 34.2g (0.2mol) 1,2,2,6,6- pentamethylpiperidinol is reacted sequentially (step and condition are identical with embodiment 1), obtain 123g of white solid [namely the bifunctional compound C shown in the following formula (C)], the yield was 93.6%.
[0090]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com