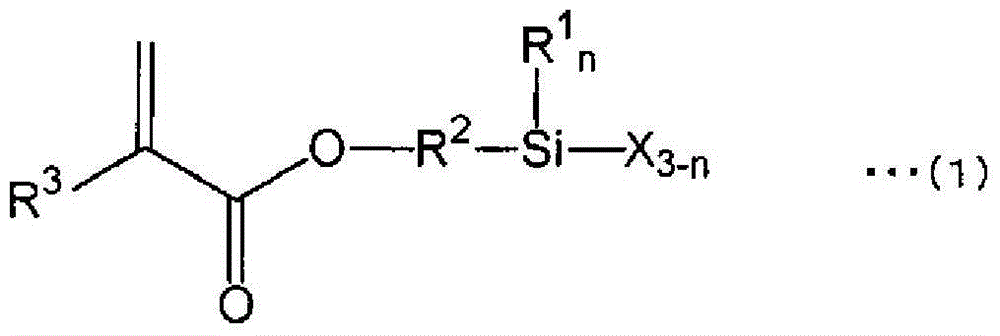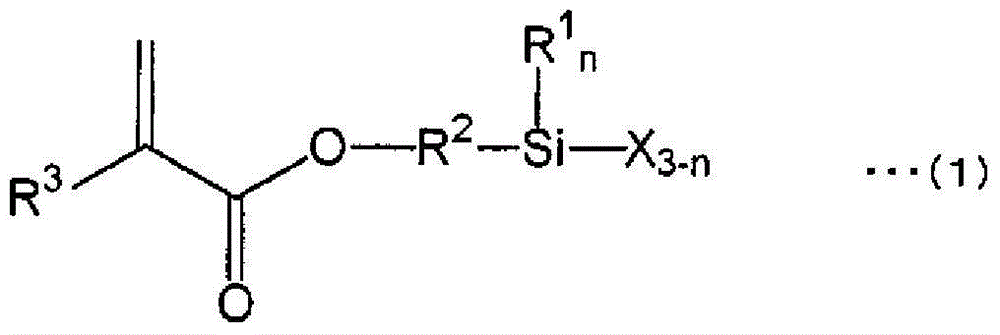Active-energy-ray-curable coating composition
An active energy ray, curing technology, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the problems of poor productivity, insufficient adhesion, deformation of the substrate, etc., to achieve high hardness, excellent scratch resistance , excellent scratch resistance and impact resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0169] Hereinafter, the present invention will be specifically described based on examples. In addition, this invention is not limited to these Examples.
[0170] In addition, "part" in the following means "weight part", and "%" means "weight%".
[0171] 1. Manufacture of component (A)
manufacture example 1
[0172] Production example 1 (manufacture of MAC-TQ)
[0173] 150 g of 1-propanol and 36.53 g (0.24 mol) of tetramethoxysilane (hereinafter referred to as "TMOS") for the alcohol exchange reaction are charged into a reactor equipped with a stirrer and a thermometer, and then they are slowly stirred while stirring. Add 4.37 g of 25% tetramethylammonium hydroxide methanol solution (0.1 mol of methanol, 12 mmol of tetramethylammonium hydroxide), and react at a temperature of 25° C. and a pH of 9 for 6 hours. Then, the internal temperature was adjusted to 60° C., and the reaction was further carried out for 1 hour while stirring. Here, the reaction liquid was analyzed by gas chromatography (TCD detector), and as a result, 1 to 4 methoxy groups contained in TMOS were detected by n-propoxy substituted 1, 2, 3, or 4 Substituted compounds and unreacted TMOS. Only trace amounts of TMOS were detected. The ratios of the n-propoxyl-containing compound (n-propoxyl-containing alkoxysilane...
manufacture example 2
[0179] Manufacturing Example 2 (Manufacturing of HDI-M305)
[0180] A mixture of pentaerythritol triacrylate (hereinafter referred to as "PETri") and pentaerythritol tetraacrylate (hereinafter referred to as "PETet") (containing PETri0.3mol and PET (0.2 mol), 159.2 g of "Aronix M-305" (trade name, hereinafter referred to as "M-305") manufactured by Toagosei Co., Ltd., 2,6-di-tert-butyl-4-methylphenol (hereinafter referred to as 0.092g of "BHT") and 0.055g of dibutyltin dilaurate (hereinafter referred to as "DBTL"), set the liquid temperature to 70-75°C, and then add hexamethylene diisocyanate dropwise while stirring them. (hereinafter referred to as "HDI") 25.2 g (0.15 mol) was reacted.
[0181] After completion|finish of dripping HDI, internal temperature was made into 80 degreeC, and reaction was continued, and it stirred for 3 hours. Subsequently, the disappearance of the isocyanate group was confirmed by IR (infrared absorption) analysis of the reaction product, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com